Parkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a neurodegenerative disease primarily of the central nervous system, affecting both motor and non-motor systems. Symptoms typically develop gradually, with non-motor issues becoming more prevalent as the disease progresses. Common motor symptoms include tremors, bradykinesia (slowness of movement), rigidity, and balance difficulties, collectively termed parkinsonism. In later stages, Parkinson's disease dementia, falls, and neuropsychiatric problems such as sleep abnormalities, psychosis, mood swings, or behavioral changes may arise.
Most cases of Parkinson's disease are sporadic, though contributing factors have been identified. Pathophysiology involves progressive degeneration of nerve cells in the substantia nigra, a midbrain region that provides dopamine to the basal ganglia, a system involved in voluntary motor control. The cause of this cell death is poorly understood but involves the aggregation of alpha-synuclein into Lewy bodies within neurons. Other potential factors involve genetic and environmental influences, medications, lifestyle, and prior health conditions.
Diagnosis is primarily based on signs and symptoms, typically motor-related, identified through neurological examination. Medical imaging techniques like neuromelanin MRI can support the diagnosis. Parkinson's typically manifests in individuals over 60, with about one percent affected. In those younger than 50, it is termed "early-onset PD".
No cure for Parkinson's is known, and treatment focuses on alleviating symptoms. Initial treatment typically includes L-DOPA, MAO-B inhibitors, or dopamine agonists. As the disease progresses, these medications become less effective and may cause involuntary muscle movements. Diet and rehabilitation therapies can help improve symptoms. Deep brain stimulation is used to manage severe motor symptoms when drugs are ineffective. There is little evidence for treatments addressing non-motor symptoms, such as sleep disturbances and mood instability. Life expectancy for those with PD is near-normal but is decreased for early-onset.
Classification and terminology
[edit]Parkinson's disease (PD) is a neurodegenerative disease affecting both the central and peripheral nervous systems, characterized by the loss of dopamine-producing neurons in the substantia nigra region of the brain.[5] It is classified as a synucleinopathy due to the abnormal accumulation of the protein alpha-synuclein, which aggregates into Lewy bodies within affected neurons.[6]
The loss of dopamine-producing neurons in the substantia nigra initially presents as movement abnormalities, leading to Parkinson's further categorization as a movement disorder.[1] In 30% of cases, disease progression leads to the cognitive decline known as Parkinson's disease dementia (PDD).[7] Alongside dementia with Lewy bodies, PDD is one of the two subtypes of Lewy body dementia.[8]
The four cardinal motor symptoms of Parkinson's—bradykinesia (slowed movements), postural instability, rigidity, and tremor—are called parkinsonism.[9][10] These four symptoms are not exclusive to Parkinson's and can occur in many other conditions,[11][12] including HIV infection and recreational drug use.[13][14] Neurodegenerative diseases that feature parkinsonism but have distinct differences are grouped under the umbrella of Parkinson-plus syndromes or, alternatively, atypical parkinsonian disorders.[15][16] Parkinson's disease can result from genetic factors or be idiopathic, in which there is no clearly identifiable cause. The latter, also called sporadic Parkinson's, makes up some 85–90% of cases.[17]
Signs and symptoms
[edit]Motor
[edit]Although a wide spectrum of motor and non-motor symptoms appear in Parkinson's, the cardinal features remain tremor, bradykinesia, rigidity, and postural instability, collectively termed parkinsonism.[18] Appearing in 70–75 percent of PD patients,[18][19] tremor is often the predominant motor symptom.[18] Resting tremor is the most common, but kinetic tremors—occurring during voluntary movements—and postural tremor—preventing upright, stable posture—also occur.[19] Tremor largely affects the hands and feet:[19] a classic parkinsonian tremor is "pill-rolling", a resting tremor in which the thumb and index finger make contact in a circular motion at 4–6 Hz frequency.[20][21]
Bradykinesia describes difficulties in motor planning, beginning, and executing, resulting in overall slowed movement with reduced amplitude that affects sequential and simultaneous tasks.[22] Bradykinesia can also lead to hypomimia, reduced facial expressions.[21] Rigidity, also called rigor, refers to a feeling of stiffness and resistance to passive stretching of muscles that occurs in up to 89 percent of cases.[23][24] Postural instability typically appears in later stages, leading to impaired balance and falls.[25] Postural instability also leads to a forward stooping posture.[26]
Beyond the cardinal four, other motor deficits, termed secondary motor symptom, commonly occur.[27] Notably, gait disturbances result in the Parkinsonian gait, which includes shuffling and paroxysmal deficits, where a normal gait is interrupted by rapid footsteps—known as festination—or sudden stops, impairing balance and causing falls.[28] [29] Most PD patients experience speech problems, including stuttering, hypophonic, "soft" speech, slurring, and festinating speech (rapid and poorly intelligible).[30] Handwriting is commonly altered in Parkinson's, decreasing in size—known as micrographia—and becoming jagged and sharply fluctuating.[31] Grip and dexterity are also impaired.[32]
Non-motor
[edit]Neuropsychiatric and cognitive
[edit]| Symptom | |
|---|---|
| Prevalence (%) | |
| Anxiety | 40–50 |
| Apathy | 40 |
| Depression | 20–40 |
| Impulse control disorders | 36–60 |
| Psychosis | 15–30 |
Neuropsychiatric symptoms like anxiety, apathy, depression, hallucinations, and impulse control disorders occur in up to 60% of those with Parkinson's. They often precede motor symptoms and vary with disease progression.[34] Non-motor fluctuations, including dysphoria, fatigue, and slowness of thinking, are also common.[35] Some neuropsychiatric symptoms are not directly caused by neurodegeneration but rather by its pharmacological management.[36]
Cognitive impairments rank among the most prevalent and debilitating non-motor symptoms.[37] These deficits may emerge in the early stages or before diagnosis,[37][38] and their prevalence and severity tend to increase with disease progression. Ranging from mild cognitive impairment to severe Parkinson's disease dementia, these impairments include executive dysfunction, slowed cognitive processing speed, and disruptions in time perception and estimation.[38]
Autonomic
[edit]
Autonomic nervous system failures, known as dysautonomia, can appear at any stage of Parkinson's.[39][40] They are among the most debilitating symptoms and greatly reduce quality of life.[41] Although almost all PD patients suffer cardiovascular autonomic dysfunction, only some are symptomatic.[41] Chiefly, orthostatic hypotension—a sustained blood pressure drop of at least 20 mmHg systolic or 10 mmHg diastolic after standing—occurs in 30–50 percent of cases. This can result in lightheadedness or fainting: subsequent falls are associated with higher morbidity and mortality.[41][42]
Other autonomic failures include gastrointestinal issues like chronic constipation, impaired stomach emptying and subsequent nausea, immoderate production of saliva, and dysphagia (difficulty swallowing): all greatly reduce quality of life.[43][44] Dysphagia, for instance, can prevent pill swallowing and lead to aspiration pneumonia.[45] Urinary incontinence, sexual dysfunction, and thermoregulatory dysfunction—including heat and cold intolerance and excessive sweating—also frequently occur.[46]
Other non-motor symptoms
[edit]Sensory deficits appear in up to 90 percent of patients and are usually present at early stages.[47] Nociceptive and neuropathic pain are common,[47] with peripheral neuropathy affecting up to 55 percent of individuals.[48] Visual impairments are also common, including deficits in visual acuity, color vision, eye coordination, and visual hallucinations.[49] An impaired sense of smell is also common.[50] PD patients can experience difficulty visually interpreting spaces and objects, as well as a reduced ability to recognize faces and emotions.[51] Difficulty reading and double vision are commonly reported.[52]
Sleep disorders are common in PD, affecting up to 98% according to a seminal 1988 study.[53] They comprise insomnia, excessive daytime sleepiness, restless legs syndrome, REM sleep behavior disorder (RBD), and sleep-disordered breathing, many of which can be worsened by medication. RBD may begin years before the initial motor symptoms. Individual presentation of symptoms varies, although most people affected by PD show an altered circadian rhythm at some point of disease progression.[54][55]
PD is also associated with a variety of skin disorders that include melanoma, seborrheic dermatitis, bullous pemphigoid, and rosacea.[56] Seborrheic dermatitis is recognized as a premotor feature that indicates dysautonomia and demonstrates that PD can be detected not only by changes of nervous tissue, but tissue abnormalities outside the nervous system as well.[57]
Causes
[edit]As of 2024, the cause of neurodegeneration in Parkinson's remains unclear,[58] though it is believed to result from the interplay of genetic and environmental factors.[58] The majority of cases are sporadic with no clearly identifiable cause, while approximately 5–10 percent are familial.[59] Around a third of familial cases can be attributed to a single monogenic cause.[59]
Molecularly, abnormal aggregation of alpha-synuclein is considered a key contributor to PD pathogenesis,[58] although the trigger for this aggregation remains debated.[60] Proteostasis disruption and the dysfunction of cell organelles, including endosomes, lysosomes, and mitochondria, are implicated in pathogenesis.[58][61] Additionally, maladaptive immune and inflammatory responses are potential contributors.[58] The substantial heterogeneity in PD presentation and progression suggests the involvement of multiple interacting triggers and pathogenic pathways.[60]
Genetic
[edit]
Parkinson's can be narrowly defined as a genetic disease, as rare inherited gene variants have been firmly established for monogenic PD, and a majority of sporadic cases carry variants that increase the risk of PD.[58][62][63] PD heritability is estimated to be between 22 and 40 percent.[58] Around 15 percent of diagnosed individuals have a family history, from which 5–10 percent can be attributed to a causative risk gene mutation, although harboring one of these mutations may not lead to disease. Rates of familial PD also vary by ethnicity: monogenic PD occurs in up to 40% of Arab-Berber patients and 20% of Ashkenazi Jewish patients.[63]
As of 2024, around 90 genetic risk variants across 78 genomic loci have been identified.[64] Notable risk genes include SNCA (which encodes alpha-synuclein), LRRK2, and VPS35 for autosomal dominant inheritance, and PRKN, PINK1, and DJ1 for autosomal recessive inheritance.[58][65] LRRK2 is the most common autosomal dominant variant and is estimated to be responsible for 1–2 percent of all cases of PD and 40 percent of familial cases.[66] [59] Parkin variants cause nearly half of recessive, early onset monogenic PD.[67] Mutations in the GBA1 gene, linked to Gaucher's disease, are found in 5–15 percent of PD cases.[68] The GBA1 variant of PD more frequently involves cognitive cognitive decline.[66]
Environmental
[edit]
The limited heritability of Parkinson's strongly implies environmental factors, but identifying these risk factors and causality is difficult due to PD's often decade-long prodromal period.[69] However, environmental toxicants such as air pollution, pesticides, and industrial solvents like trichloroethylene are strongly linked to Parkinson's.[70]
Certain pesticides—like paraquat, glyphosate, and rotenone—are the most established environmental toxicants for Parkinson's, and are likely causal.[71][72][73] PD prevalence is strongly associated with local pesticide use, and many pesticides harm mitochondria.[74] Paraquat, for instance, structurally resembles metabolized MPTP,[71] which selectively kills dopaminergic neurons by inhibiting mitochondrial complex 1 and is widely used to model PD.[75][71] Pesticide exposure after diagnosis may also accelerate disease progression.[71] Without pesticide exposure, an estimated 20 percent of all PD cases would be prevented.[76]
Hypotheses
[edit]Prionic hypothesis
[edit]The hallmark of Parkinson's is the formation of protein aggregates, initially alpha-synuclein fibrils and then Lewy bodies and Lewy neurites.[77] The prion hypothesis holds that alpha-synuclein aggregates are pathogenic: they can travel to neighboring, healthy neurons and seed new aggregates. Some suggest that the heterogeneity of PD may be due to different "strains" of alpha-synuclein aggregates and different anatomical sites of origin.[78][79] Alpha-synuclein propagation has been demonstrated in cell and animal models and is the most popular explanation for the progressive spread through specific neuronal systems.[80] However, therapeutic efforts to clear alpha-synuclein have failed.[81] Additionally, postmortem brain tissue analysis shows that alpha-synuclein pathology does not progress through clearly progress through the nearest neural connections.[82]
Braak's hypothesis
[edit]In 2002, Heiko Braak and colleagues proposed that Parkinson's begins outside the brain and is caused by a "neuroinvasion" of some unknown pathogen.[83][84] The pathogen enters through the nasal cavity and is swallowed into the digestive tract, initiating Lewy pathology in both areas.[73][83] This alpha-synuclein pathology can then travel from the gut to the central nervous system through the vagus nerve.[85] This might explain the presence of Lewy pathology in both the enteric nervous system and olfactory tract neurons, as well as clinical symptoms like loss of small and gastrointestinal problems.[84] It has also been suggested that environmental toxicants might be similarly ingested to trigger PD.[86]
Catecholaldehyde hypothesis
[edit]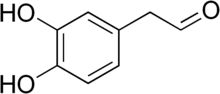
The enzyme monoamine oxidase (MAO) play a central role in the metabolism of the neurotransmitter dopamine and other catecholamines. The catecholaldehyde hypothesis argues that the oxidation of dopamine by MAO into 3,4-dihydroxyphenylacetaldehyde (DOPAL) and hydrogen peroxide and the subsequent abnormal accumulation thereof leads to neurodegeneration. The theory posits that DOPAL interacts with alpha-synuclein and causes it to aggregate.[87][88]
Mitochondrial dysfunction
[edit]Whether mitochondrial dysfunction is a cause or consequence of PD pathology remains unclear.[89] Impaired ATP production, increased oxidative stress, and reduced calcium buffering may contribute to neurodegeneration.[90] The finding that MPP+—a respiratory complex I inhibitor and MPTP metabolite—caused parkinsonian symptoms strongly implied that mitochondria contributed to PD pathogenesis.[91][92] Alpha synuclein and toxicants like rotenone similarly disrupt respiratory complex I.[93] Additionally, faulty genes variants involved in familial Parkinson's—including PINK1 and Parkin—prevent the elimination of dysfunctional mitochondria through mitophagy.[94][95]
Neuroinflammation
[edit]Some hypothesize that neurodegeneration arises from a chronic neuroinflammatory state created by local activated microglia and infiltrating immune cells.[58] Mitochondrial dysfunction may also drive immune activation, particularly in monogenic PD.[58] Some autoimmune disorders increase the risk of developing PD, supporting an autoimmune contribution.[96] Additionally, influenza and herpes simplex virus infections increase the risk of PD, possibly due to a viral protein resembling alpha-synuclein.[97] Parkinson's risk is also decreased with immunosuppressants.[58]
Pathophysiology
[edit]
Parkinson's disease has two hallmark pathophysiological processes: the abnormal aggregation of alpha-synuclein that leads to Lewy pathology, and the degeneration of dopaminergic neurons in the substantia nigra pars compacta.[98][99] The death of these neurons reduces available dopamine in the striatum, which in turn affects circuits controlling movement in the basal ganglia.[99] By the time motor symptoms appear, 50–80 percent of all dopaminergic neurons in the substantia nigra have degenerated.[99]
However, cell death and Lewy pathology are not limited to the substantia nigra.[100] The six-stage Braak system holds that alpha-synuclein pathology begins in the olfactory bulb or outside the central nervous system in the enteric nervous system before ascending the brain stem.[101] In the third Braak stage, lewy body pathology appears in the substantia nigra,[101] and, by the sixth step, lewy pathology has spread to the limbic and neocortical regions.[102] Although Braak staging offers a strong basis for PD progression, the Lewy pathology around 50 percent patients do not adhere to the predicted model.[103] Indeed, lewy pathology is highly variable and may be entirely absent in some PD patients.[100][104]
Alpha synuclein pathology
[edit]
Alpha-synuclein is an intracellular protein typically localized to presynaptic terminals and involved in synaptic vesicle trafficking, intracellular transport, and neurotransmitter release.[103][105] When misfolded, it can aggregate into oligomers and proto-fibrils that in turn lead to Lewy body formation.[105][106][107] Due to their lower molecular weight, oligomers and proto-fibrils may disseminate and be transmitted to other cells more rapidly.[107]
Lewy bodies consist of a fibrillar exterior and granular core. Although alpha-synuclein is the dominant proteinaceous component, the core contains mitochondrial and autophagosomal membrane components, suggesting a link with organelle dysfunction.[108][109] It is unclear whether Lewy bodies themselves contribute to or are simply the result of PD pathogenesis: alpha-synuclein oligomers can independently mediate cell damage, and neurodegeneration can precede Lewy body formation.[110]
Pathways involved in neurodegeneration
[edit]Three major pathways—vesicular trafficking, lysosomal degradation, and mitochondrial maintenance—are known to be affected by and contribute to Parkinson's pathogenesis, with all three linked to alpha-synuclein.[111] High risk gene variants also impair all three of these processes.[111] All steps of vesicular trafficking are impaired by alpha-synuclein. It blocks endoplasmic reticulum (ER) vesicles from reaching the Golgi—leading to ER stress—and Golgi vesicles from reaching the lysosome, preventing alpha synuclein degradation and leading to its build-up.[112] Risky gene variants, chiefly GBA, further compromise lysosomal function.[113] Although the mechanism is not well established, alpha-synuclein can impair mitochondrial function and cause subsequent oxidative stress. Mitochondrial dysfunction can in turn lead to further alpha-synuclein accumulation in a positive feedback loop.[114] Microglial activation, possibly caused by alpha-synuclein, is also strongly indicated.[115][116]
Risk factors
[edit]Positive risk factors
[edit]As 90 percent of Parkinson's cases are sporadic, the identification of the risk factors that may influence disease progression or severity is critical.[117][69] The most significant risk factor in developing PD is age, with a prevalence of 1 percent in those aged over 65 and approximately 4.3 percent in age over 85.[118] Traumatic brain injury significant increases PD risk, especially if recent.[119][120] Dairy consumption is associated with a higher risk, possibly due to contaminants like heptachlor epoxide.[121] Although the connection is unclear, melanoma diagnosis is associated with an approximately 45 percent risk increase.[121] There is also an association between methamphetamine use and PD risk.[121]
Negative risk factors
[edit]
Although no compounds or activities have been mechanistically established as neuroprotective for Parkinson's,[122][123] several factors have been found to be associated with a decreased risk.[122] Tobacco use and smoking is strongly associated with a decreased risk, reducing the chance of developing PD by up to 70%.[124][125][121] Various tobacco and smoke components have been hypothesized to be neuroprotective, including nicotine, carbon monoxide, and monoamine oxidase B inhibitors.[126][127] Consumption of coffee, tea, or caffeine is also strongly associated with neuroprotection.[128][129] Prescribed adrenergic antagonists like terazosin may reduce risk.[128]
Although findings have varied, usage of nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen may be neuroprotective.[130][131] Calcium channel blockers may also have a protective effect, with a 22% risk reduction reported.[132] Higher blood concentrations of urate—a potent antioxidant—have been proposed to be neuroprotective.[126][133] Although longitudinal studies observe a slight decrease in PD risk among those who consume alcohol—possibly due to alcohol's urate-increasing effect—alcohol abuse may increase risk.[134][135]
Diagnosis
[edit]
A physician's initial assessment is typically based on medical history and neurological examination.[136] They assess motor symptoms (bradykinesia, rest tremors, etc.) using clinical diagnostic criteria. The finding of Lewy bodies in the midbrain on autopsy is usually considered final proof that the person had PD. The clinical course of the illness over time may diverge from PD, requiring that presentation is periodically reviewed to confirm the accuracy of the diagnosis.[136][137]
Multiple causes can occur for Parkinsonism or diseases that look similar. Stroke, certain medications, and toxins can cause "secondary parkinsonism" and need to be assessed during a visit.[138][137] Parkinson-plus syndromes, such as progressive supranuclear palsy and multiple system atrophy, must be considered and ruled out appropriately to begin a different treatment and disease progression (anti-Parkinson's medications are typically less effective at controlling symptoms in Parkinson-plus syndromes).[136] Faster progression rates, early cognitive dysfunction or postural instability, minimal tremor, or symmetry at onset may indicate a Parkinson-plus disease rather than PD itself.[139]
Medical organizations have created diagnostic criteria to ease and standardize the diagnostic process, especially in the early stages of the disease. The most widely known criteria come from the UK Queen Square Brain Bank for Neurological Disorders and the U.S. National Institute of Neurological Disorders and Stroke. The Queen Square Brain Bank criteria require slowness of movement (bradykinesia) plus either rigidity, resting tremor, or postural instability. Other possible causes of these symptoms need to be ruled out. Finally, three or more of the following supportive symptoms are required during onset or evolution: unilateral onset, tremor at rest, progression in time, asymmetry of motor symptoms, response to levodopa for at least five years, the clinical course of at least ten years and appearance of dyskinesias induced by the intake of excessive levodopa.[140] If a suspected case of PD does not respond to levodopa then the diagnosis should be reconsidered.[141] Assessment of sudomotor function through electrochemical skin conductance can be helpful in diagnosing dysautonomia.[142]
When PD diagnoses are checked by autopsy, movement disorders experts are found on average to be 79.6% accurate at initial assessment and 83.9% accurate after refining diagnoses at follow-up examinations. When clinical diagnoses performed mainly by nonexperts are checked by autopsy, the average accuracy is 73.8%. Overall, 80.6% of PD diagnoses are accurate, and 82.7% of diagnoses using the Brain Bank criteria are accurate.[143]
Imaging
[edit]Computed tomography (CT) scans of people with PD usually appear normal.[144] Magnetic resonance imaging has become more accurate in diagnosis of the disease over time, specifically through iron-sensitive T2* and susceptibility weighted imaging sequences at a magnetic field strength of at least 3T, both of which can demonstrate absence of the characteristic 'swallow tail' imaging pattern in the dorsolateral substantia nigra.[145] In a meta-analysis, absence of this pattern was highly sensitive and specific for the disease.[146] A meta-analysis found that neuromelanin-MRI can discriminate individuals with Parkinson's from healthy subjects.[147] Diffusion MRI has shown potential in distinguishing between PD and Parkinson-plus syndromes, as well as between PD motor subtypes,[148] though its diagnostic value is still under investigation.[144] CT and MRI are used to rule out other diseases that can be secondary causes of parkinsonism, most commonly encephalitis and chronic ischemic insults, as well as less-frequent entities such as basal ganglia tumors and hydrocephalus.[144]
The metabolic activity of dopamine transporters in the basal ganglia can be directly measured with positron emission tomography and single-photon emission computed tomography scans. It has shown high agreement with clinical diagnoses of PD.[149] Reduced dopamine-related activity in the basal ganglia can help exclude drug-induced Parkinsonism. This finding is nonspecific and can be seen with both PD and Parkinson-plus disorders.[144] In the United States, DaTSCANs are only FDA approved to distinguish PD or Parkinsonian syndromes from essential tremor.[150]
Iodine-123-meta-iodobenzylguanidine myocardial scintigraphy can help locate denervation of the muscles of the heart which can support a PD diagnosis.[151]
Differential diagnosis
[edit]Secondary parkinsonism – The multiple causes of parkinsonism can be differentiated through careful history, physical examination, and appropriate imaging.[151][152] Other Parkinson-plus syndromes can have similar movement symptoms but have a variety of associated symptoms. Some of these are also synucleinopathies. Lewy body dementia involves motor symptoms with early onset of cognitive dysfunction and hallucinations that precede motor symptoms. Alternatively, multiple systems atrophy or MSA usually has early onset of autonomic dysfunction (such as orthostasis), and may have autonomic predominance, cerebellar symptom predominance, or Parkinsonian predominance.[153]
Other Parkinson-plus syndromes involve tau, rather than alpha-synuclein. These include progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS). PSP predominantly involves rigidity, early falls, bulbar symptoms, and vertical gaze restriction; it can be associated with frontotemporal dementia symptoms. CBS involves asymmetric parkinsonism, dystonia, alien limb, and myoclonic jerking.[154] Presentation timelines and associated symptoms can help differentiate similar movement disorders from idiopathic Parkinson disease.[medical citation needed]
Vascular parkinsonism is the phenomenon of the presence of Parkinson's disease symptoms combined with findings of vascular events (such as a cerebral stroke). The damaging of the dopaminergic pathways is similar in cause for both vascular parkinsonism and idiopathic PD and present with similar symptoms. Differentiation can be made with careful bedside examination, history evaluation, and imaging.[155][156]
Parkinson-plus syndrome – Multiple diseases can be considered part of the Parkinson's plus group, including corticobasal syndrome, multiple system atrophy, progressive supranuclear palsy, and dementia with Lewy bodies. Differential diagnosis can be narrowed down with careful history and physical exam (especially focused on the sequential onset of specific symptoms), progression of the disease, and response to treatment.[157][152] Some key symptoms:[158][152]
- Corticobasal syndrome – levodopa-resistance, myoclonus, dystonia, corticosensory loss, apraxia, and non-fluent aphasia
- Dementia with Lewy bodies – levodopa resistance, cognitive predominance before motor symptoms, and fluctuating cognitive symptoms, (visual hallucinations are common in this disease)
- Essential tremor – This can at first look like Parkinsonism, but has key differentiators. In essential tremor, the tremor gets worse with action (improves in PD), a lack of other symptoms is common in PD, and normal DatSCAN is seen.[152]
- Multiple system atrophy – levodopa resistance, rapidly progressive, autonomic failure, stridor, present Babinski sign, cerebellar ataxia, and specific MRI findings
- Progressive supranuclear palsy – levodopa resistance, restrictive vertical gaze, specific MRI findings, and early and different postural difficulties
Management
[edit]As of 2024, no disease-modifying therapies exist that reverse or slow neurodegeneration, processes respectively termed neurorestoration and neuroprotection.[122][123] Patients are typically managed with a holistic approach that combines lifestyle modifications with physical therapy.[159] Current pharmacological interventions purely target symptoms, by either increasing endogenous dopamine levels or directly mimicking dopamine's effect on the patient's brain.[160][159] These include dopamine agonists, MAO-B inhibitors, and levodopa: the most widely used and effective drug.[161][159] The optimal time to initiate pharamacological treatment is debated,[162] but initial dopamine agonist and MAO-B inhibitor treatment and later levodopa therapy is common.[163] Invasive procedures such as deep brain stimulation may be used for patients that do not respond to medication.[164][165]
Medications
[edit]Levodopa
[edit]
Levodopa (L-DOPA) is the most widely used and the most effective therapy—the gold standard—for Parkinson's treatment.[161] The compound occurs naturally and is the immediate precursor for dopamine synthesis in the dopaminergic neurons of the substantia nigra.[166] Levodopa administration reduces the dopamine deficiency, decreasing parkisonian symptoms.[167][168]
Despite its efficacy, levodopa poses several challenges and has been called the "pharmacologist's nightmare".[169][170] Its metabolism outside the brain by aromatic L-amino acid decarboxylase (AAAD) and catechol-O-methyltransferase (COMT) can cause nausea and vomiting; inhibitors like carbidopa, entacapone, and benserazide are usually taken with levodopa to mitigate these effects.[171][172][note 1] Symptoms may become unresponsive to levodopa, with sudden changes between a state of mobility ("ON time") and immobility ("OFF time").[174] Longterm levodopa use may also induce dyskinesia and motor fluctuations. Although this often causes levodopa use to be delayed to later stages, earlier administration leads to improved motor function and quality of life.[175]
Dopamine agonists
[edit]Dopamine agonists are an alternative or complement for levodopa therapy. They activate dopamine receptors in the striatum, with reduced risk of motor fluctuations and dyskinesia.[176] Ergot dopamine agonists were commonly used, but have been largely replaced with non-ergot compounds due to severe adverse effects like pulmonary fibrosis and cardiovascular issues.[176] Non-ergot agonists are efficacious in both early and late stage Parkinson's,[177] The agonist apomorphine is often used for drug-resistant OFF time in later-stage PD.[177][178] However, after five years of use, impulse control disorders may occur in over 40 percent of PD patients taking dopamine agonists.[162] A problematic, narcotic-like withdrawal effect may occur when agonist use is reduced or stopped.[162][179] Compared to levodopa, dopamine agonists are more likely to cause fatigue, daytime sleepiness, and hallucinations.[179]
MAO-B inhibitors
[edit]MAO-B inhibitors—such as safinamide, selegiline and rasagiline—increase the amount of dopamine in the basal ganglia by inhibiting the activity of monoamine oxidase B, an enzyme that breaks down dopamine.[180] These compounds mildly alleviate motor symptoms when used as monotherapy but can also be used with levodopa and can be used at any disease stage.[181] When used with levodopa, time spent in the off phase is reduced.[182][183] Selegiline has been shown to delay the need for initial levodopa, suggesting that it might be neuroprotective and slow the progression of the disease.[184] Common side effects are nausea, dizziness, insomnia, sleepiness, and (in selegiline and rasagiline) orthostatic hypotension.[184][138] MAO-Bs are known to increase serotonin and cause a potentially dangerous condition known as serotonin syndrome.[184][185]
Other drugs
[edit]Treatments for non-motor symptoms of PD have not been well studied and many medications are used off-label.[66] A diverse range of symptoms beyond those related to motor function can be treated pharmaceutically.[186] Examples include cholinesterase inhibitors for cognitive impairment and modafinil for excessive daytime sleepiness.[187] Fludrocortisone, midodrine and droxidopa are commonly used off label for orthostatic hypotension related to autonomic dysfunction. Sublingual atropine or botulinum toxin injections may be used off-label for drooling. SSRIs and SNRIs are often used for depression related to PD, but there is a risk of serotonin syndrome with the SSRI or SNRI antidepressants.[66] Doxepin and rasagline may reduce physical fatigue in PD.[188] Other treaments have received government approval, such as the first FDA-approved treatment for PD psychosis, pimavanserin. Although its efficacy is inferior to off-label clozapine, it has significantly fewer side effects.[189]
Invasive interventions
[edit]
Surgery for Parkinson's first appeared in the 19th century and by the 1960s had evolved into ablative brain surgery that lesioned the basal ganglia, thalamus or globus pallidus (a pallidotomy).[190] The discovery of L-DOPA for PD treament caused ablative therapies to largely disappear.[191][192] Ablative surgeries experienced a resurgence in the 1990s but were quickly superseded by newly-developed deep brain stimulation (DBS).[192] Although gamma knife and high-intensity focused ultrasound surgeries have been developed for pallidotomies and thalamotomies, their use remains rare.[193][194]
DBS involves the implantation of electrodes called neurostimulators, which sends electrical impulses to specific parts of the brain.[164] DBS for the subthalamic nucleus and globus pallidus interna has high efficacy for up to 2 years, but longterm efficacy is unclear and likely decreases with time.[164] DBS typically targets rigidity and tremor,[195] and is recommended for PD patients who are intolerant or do not respond to medication.[165] Cognitive impairment is the most common exclusion criteria.[196]
Rehabilitation
[edit]
Although pharmacological therapies can improve symptoms, patients' autonomy and ability to perform everyday tasks is still reduced by PD. As a result, rehabilitation is often useful. However, the scientific support for any single rehabilitation treatment is limited.[197]
Exercise programs are often recommended, with preliminary evidence of efficacy.[198][199][200] Regular physical exercise with or without physical therapy can be beneficial to maintain and improve mobility, flexibility, strength, gait speed, and quality of life.[198] Aerobic, mind-body, and resistance training may be beneficial in alleviating PD-associated depression and anxiety.[200][201] Strength training may increase manual dexterity and strength, facilitating daily tasks that require grasping objects.[202]
In improving flexibility and range of motion for people experiencing rigidity, generalized relaxation techniques such as gentle rocking have been found to decrease excessive muscle tension. Other effective techniques to promote relaxation include slow rotational movements of the extremities and trunk, rhythmic initiation, diaphragmatic breathing, and meditation.[203] Deep diaphragmatic breathing may also improve chest-wall mobility and vital capacity decreased by the stooped posture and respiratory dysfunctions of advanced Parkinson's.[204] Rehabilitation techniques targeting gait and the challenges posed by braykinesia, shuffling, and decreased arm swing include pole walking, treadmill walking, and marching exercises.[205]
Speech therapies such as the Lee Silverman voice treatment may reduce the effect of speech disorders associated with PD.[206][207] Occupational therapy is another rehabilitation strategy and can improve quality of life by enabling PD patients to find engaging activities and communal roles, adapt to their living enviornment, and improving domestic and work abilities.[208]
Diet
[edit]Parkinson's poses digestive problems like constipation and prolonged emptying of stomach contents, and a balanced diet with periodical nutritional assessments is recommended to avoid weight loss or gain and minimize the consequences of gastrointestinal dysfunction. In particular, a Mediterranean diet is advised and may slow disease progression.[209][210] As it can compete for uptake with amino acids derived from protein, levodopa should be taken 30 minutes before meals to minimize such competition. Low protein diets may also be needed by later stages.[210] As the disease advances, swallowing difficulties often arise. Using thickening agents for liquid intake and an upright posture when eating may be useful; both measures reduce the risk of choking. Gastrostomy can be used to deliver food directly into the stomach.[211][212] Increased water and fiber intake is used to treat constipation.[213]
Palliative care
[edit]As Parkinson's is incurable, palliative care aims to improve the quality of life for both the patient and family by alleviating the symptoms and stress associated with illness.[214][215][216] Early integration of palliative care into the disease course is recommended, rather than delaying until later stages.[214] Palliative care specialists can help with physical symptoms, emotional factors such as loss of function and jobs, depression, fear, as well as existential concerns.[217] Palliative care team members also help guide patients and families on difficult decisions caused by disease progression, such as wishes for a feeding tube, noninvasive ventilator or tracheostomy, use of cardiopulmonary resuscitation, and entering hospice care.[218][219]
Prognosis
[edit]| Parkinson's subtype | Mean years post-diagnosis until: | |
|---|---|---|
| Severe cognitive or movement abnormalities[note 2] | Death | |
| Mild-motor predominant | 14.3 | 20.2 |
| Intermediate | 8.2 | 13.1 |
| Diffuse malignant | 3.5 | 8.1 |
As Parkinson's is a heterogeneous condition with multiple etiologies, prognostication can be difficult and prognoses can be highly variable.[220][222] On average, life expectancy is reduced in those with Parkinson's, with younger age of onset resulting in greater life expectancy decreases.[223] Although PD subtype categorization is controversial, the 2017 Parkinson's Progression Markers Initiative study identified three broad scorable subtypes of increasing severity and more rapid progression: mild-motor predominant, intermediate, and diffuse malignant. Mean years of survival post-diagnosis were 20.2, 13.1, and 8.1.[220]
Around 30% of Parkinson's patients develop dementia, and is 12 times more likely to occur in elderly patients of those with severe PD.[224] Dementia is less likely to arise in patients with tremor-dominant PD.[225] Parkinson's disease dementia is associated with a reduced quality of life in people with PD and their caregivers, increased mortality, and a higher probability of needing nursing home care.[226]
The incidence rate of falls in Parkinson's patients is approximately 45 to 68%, thrice that of healthy individuals, and half of such falls result in serious secondary injuries. Falls increase morbidity and mortality.[227] Around 90% of those with PD develop hypokinetic dysarthria, which worsens with disease progression and can hinder communication.[228] Additionally, over 80% of PD patients develop dysphagia: consequent inhalation of gastric and oropharyngeal secretions can lead to aspiration pneumonia.[229] Aspiration pneumonia is responsible for 70% of deaths in those with PD.[230]
Epidemiology
[edit]
As of 2024, Parkinson's is the second most common neurodegenerative disease and the fastest-growing in total number of cases.[231][232] As of 2023, global prevalence was estimated to be 1.51 per 1000.[233] Although it is around 40% more common in men,[234] age is the dominant predeterminant of Parkinson's.[235] Consequently, as global life expectancy has increased, Parkinson's disease prevalence has also risen, with an estimated increase in cases by 74% from 1990 to 2016.[236] The total number is predicted to rise to over 12 million patients by 2040.[237] Some label this a pandemic.[236]
This increase may be due to a number of global factors, including prolonged life expectancy, increased industrialisation, and decreased smoking.[236] Although genetics is the sole factor in a minority of cases, most cases of Parkinson's are likely a result of gene-environment interactions: concordance studies with twins have found Parkinson's heritability to be just 30%.[234] The influence of multiple genetic and environmental factors complicates epidemiological efforts.[238]
Relative to Europe and North America, disease prevalence is lower in Africa but similar in Latin America.[239] Although China is predicted to have nearly half of the global Parkinson's population by 2030,[240] estimates of prevalence in Asia vary.[239] Potential explanations for these geographic differences include genetic variation, environmental factors, health care access, and life expectancy.[239] Although PD incidence and prevalence may vary by race and ethnicity, significant disparities in care, diagnosis, and study participation limit generalizability and lead to conflicting results.[239][238] Within the United States, high rates of PD have been identified in the Midwest, the South, and agricultural regions of other states: collectively termed the "PD belt".[241] The association between rural residence and Parkinson's has been hypothesized to be caused by environmental factors like herbicides, pesticides, and industrial waste.[241][242]
History
[edit]In 1817, English physician James Parkinson published the first full medical description of the disease as a neurological syndrome in his monograph An Essay on the Shaking Palsy.[244][245] He presented six clinical cases, including three he had observed on the streets near Hoxton Square in London.[246] Parkinson described three cardinal symptoms: tremor, postural instability and "paralysis" (undistinguished from rigidity or bradykinesia), and speculated that the disease was caused by trauma to the spinal cord.[247][248]
There was little discussion or investigation of the "shaking palsy" until 1861, when Frenchman Jean-Martin Charcot—regarded as the father of neurology—began expanding Parkinson's description, adding bradykinesia as one of the four cardinal symptoms.[247][246][248] In 1877, Charcot renamed the disease after Parkinson, as not all patients displayed the tremor suggested by "shaking palsy".[246][248] Subsequent neurologists who made early advances to the understanding of Parkinson's include Armand Trousseau, William Gowers, Samuel Kinnier Wilson, and Wilhelm Erb.[249]

Although Parkinson is typically credited with the first detailed description of PD, many previous texts reference some of the disease's clinical signs.[250] In his essay, Parkinson himself acknowledged partial descriptions by Galen, William Cullen, Johann Juncker, and others.[248] Possible earlier but incomplete descriptions include a Nineteenth Dynasty Egyptian papyrus, the ayurvedic text Charaka Samhita, Ecclesiastes 12:3, and a discussion of tremors by Leonardo da Vinci.[248][251] Multiple traditional Chinese medicine texts may include references to PD, including a discussion in the Yellow Emperor's Internal Classic (c. 425–221 BC) of a disease with symptoms of tremor, stiffness, staring, and stooped posture.[251] In 2009, a systematic description of PD was found in the Hungarian medical text Pax corporis written by Ferenc Pápai Páriz in 1690, some 120 years before Parkinson. Although Páriz correctly described all four cardinal signs, it was only published in Hungarian and was not widely distributed.[252][253]
In 1912, Frederic Lewy described microscopic particles in affected brains, later named Lewy bodies.[254] In 1919, Konstantin Tretiakoff reported that the substantia nigra was the main brain structure affected, corroborated by Rolf Hassler in 1938.[255] The underlying changes in dopamine signaling were identified in the 1950s, largely by Arvid Carlsson and Oleh Hornykiewicz.[256] In 1997, Polymeropoulos and colleagues at the NIH discovered the first gene for PD,[257] SNCA, which encodes alpha-synuclein. Alpha-synuclein was in turn found to be the main component of Lewy bodies by Spillantini, Trojanowski, Goedert, and others.[258] Anticholinergics and surgery were the only treatments until the use of levodopa,[259][260] which, although first synthesized by Casimir Funk in 1911,[261] did not enter clinical use until 1967.[262] By the late 1980s, deep brain stimulation introduced by Alim Louis Benabid and colleagues at Grenoble, France, emerged as an additional treatment.[263]
Society and culture
[edit]
Social impact
[edit]For some people with PD, masked facial expressions and difficulty moderating facial expressions of emotion or recognizing other people's facial expressions can impact social well-being.[264] As the condition progresses, tremor, other motor symptoms, difficulty communicating, or mobility issues may interfere with social engagement, causing individuals with PD to feel isolated.[265] Public perception and awareness of PD symptoms such as shaking, hallucinating, slurring speech, and being off balance is lacking in some countries and can lead to stigma.[266]
Cost
[edit]The economic cost of Parkinson's to both individuals and society is high.[267] Globally, most government health insurance plans do not cover Parkinson's therapies, requiring patients to pay out-of-pocket.[267] Indirect costs include lifetime earnings losses due to premature death, productivity losses, and caregiver burdens.[268] The duration and progessive nature of PD can place a heavy burden on caregivers:[269] family members like spouses dedicate around 22 hours per week to care.[268]
In 2010, the total economic burden of Parkinson's across Europe, including indirect and direct medical costs, was estimated to be €13.9 billion (US $14.9 billion) in 2010.[270] The total burden in the United States was estimated to be $51.9 billion in 2017, and is project to surpass $79 billion by 2037.[268] However, as of 2022, no rigorous economic surveys had been performed for low or middle income nations.[271] Regardless, preventative care has been identified as crucial to prevent the rapidly increasing incidence of Parkinson's from overwhelming national health systems.[269]
Advocacy
[edit]
The birthday of James Parkinson, 11 April, has been designated as World Parkinson's Day.[272] A red tulip was chosen by international organizations as the symbol of the disease in 2005; it represents the 'James Parkinson' tulip cultivar, registered in 1981 by a Dutch horticulturalist.[273]
Advocacy organizations include the National Parkinson Foundation, which has provided more than $180 million in care, research, and support services since 1982,[274] Parkinson's Disease Foundation, which has distributed more than $115 million for research and nearly $50 million for education and advocacy programs since its founding in 1957 by William Black;[275][276] the American Parkinson Disease Association, founded in 1961;[277] and the European Parkinson's Disease Association, founded in 1992.[278]
Notable cases
[edit]
In the 21st century, the diagnosis of Parkinson's among notable figures has increased the public's understanding of the disorder.[279] Actor Michael J. Fox was diagnosed with PD at 29 years old,[280] and has used his diagnosis to increase awareness of the disease.[281] To illustrate the effects of the disease, Fox has appeared without medication in television roles and before the United States Congress without medication.[282] The Michael J. Fox Foundation, which he founded in 2000, has raised over $2 billion for Parkinson's research.[283]
Boxer Muhammad Ali showed signs of PD when he was 38, but was undiagnosed until he was 42, and has been called the "world's most famous Parkinson's patient". [284] Whether he had PD or parkinsonism related to boxing is unresolved.[285] Cyclist and Olympic medalist Davis Phinney, diagnosed with Parkinson's at 40, started the Davis Phinney Foundation in 2004 to support PD research.[286][287]
Several historical figures have been theorized to have had Parkinson's, often framed in the industriousness and inflexibility of the so-called "Parkinsonian personality".[288][289] For instance, English philosopher Thomas Hobbes was diagnosed with "shaking palsy"—assumed to have been Parkinson's—but continued writing works such as Leviathan.[290][291][292] Adolf Hitler is widely believed to have had Parkinson's, and the condition may have influenced his decision making.[293][294][295] Mao Zedong was also reported to have died from the disorder.[296]
Clinical research
[edit]
As of 2024, no disease-modifying therapies exist that reverse or slow the progression of Parkinson's.[122][123] Active research directions include the search for new animal models of the disease and studies of the potential usefulness of gene therapy, stem cell transplants, and neuroprotective agents.[297] Improved treatments will likely use a combination of therapeutic strategies to improve PD symptoms and maximize outcomes.[298] Reliable biomarkers for Parkinson's are also needed for early diagnosis.[299] Research criteria for their identification have been established.[300]
Neuroprotective treatments
[edit]Anti-alpha-synuclein drugs that prevent alpha-synuclein oligomerization and aggregation or promote their clearance are being heavily explored, and potential therapeutic strategies include small molecules and immunotherapies like vaccines and monoclonal antibodies.[301][302][303] Although immunotherapies have shown promise, their effiacy is often inconsistent.[302] Anti-inflammatory drugs that target NLRP3 and the JAK-STAT signaling pathway are another possible therapeutic strategy.[304]
As the gut microbiome in PD is often disrupted and may produce toxic compounds, fecal microbiota transplants may restore a healthy microbiome and improve various motor and non-motor symptoms.[301] Although neurotrophic factors—peptides that enhance the growth, maturation, and survival of neurons—have shown modest results and require invasive surgical administration, less invasive routes such as viral vectors are being explored.[305] Calcium channel blockers may restore the calcium imbalance present in Parkinson's, and are being investigated as a neuroprotective treatment.[306] Other therapies, like deferiprone, may reduce the abnormal accumulation of iron in PD.[306]
Cell-based therapies
[edit]In contrast to other neurodegenerative disorders, many Parkinson's symptoms can be attributed to the loss of a single cell type. Consequently, dopaminergic neuron regeneration is a promising therapeutic approach.[307] Although most initial research sought to generate dopaminergic neuron precursor cells from fetal brain tissue,[308] pluripotent stem cells—particularly induced pluripotent stem cells (iPSCs)—have become an increasingly popular tissue source.[309][310]
Both fetal and iPSC-derived DA neurons have been transplanted into patients in clinical trials.[311][312] Although some patients see improvements, the results are highly variable. Adverse effects, such as dyskinesia arising from excess dopamine release by the transplanted tissues, have also been observed.[313][314]
Gene therapy
[edit]Gene therapy for Parkinson's seeks to restore the healthy function of dopaminergic neurons in the substantia nigra by delivering genetic material—typically through a viral vector—to these diseased cells.[315][316] This material may deilver a functional, wildtype version of a gene, or knockdown a pathological variants.[317] Experimental gene therapies for PD have aimed to increase the expression of growth factors or enzymes involved in dopamine synthesis, like tyrosine hydroxylase.[318] The one-time delivery of genes circumvents the recurrent invasive administration required to administer some peptides and proteins to the brain.[319] MicroRNAs are an emerging PD gene therapy platform that serve as an alternative to viral vectors.[320]
Notes and references
[edit]Notes
[edit]- ^ These inhibitors do not cross the blood brain barrier and thus do not prevent levodopa metabolism there.[173]
- ^ Defined as the onset of development of recurrent falls, wheelchair dependence, dementia, or facility placement.[220]
Citations
[edit]- ^ a b National Institute of Neurological Disorders and Stroke.
- ^ Ferri 2010, Chapter P.
- ^ Koh & Ito 2017.
- ^ Ou et al. 2021.
- ^ Ramesh & Arachchige 2023, pp. 200–201, 203.
- ^ Calabresi et al. 2023, pp. 1, 5.
- ^ Wallace et al. 2021, p. 149.
- ^ Hansen et al. 2019, p. 635.
- ^ Bhattacharyya 2017, p. 7.
- ^ Stanford University School Medicine.
- ^ Bologna, Truong & Jankovic 2022, pp. 1–6.
- ^ Limphaibool et al. 2019, pp. 1–2.
- ^ Leta et al. 2022, p. 1122.
- ^ Langston 2017, p. S11.
- ^ Prajjwal et al. 2024, pp. 1–3.
- ^ Olfatia, Shoeibia & Litvanb 2019, p. 101.
- ^ Dolgacheva, Zinchenko & Goncharov 2022, p. 2.
- ^ a b c Abusrair, Elsekaily & Bohlega 2022, p. 2.
- ^ a b c Moustafa et al. 2016, p. 730.
- ^ Abusrair, Elsekaily & Bohlega 2022, p. 4.
- ^ a b Sveinbjornsdottir 2016, p. 319.
- ^ Bologna et al. 2020, pp. 727–729.
- ^ Ferreira-Sánchez, Moreno-Verdú & Cano-de-la-Cuerda 2020, p. 1.
- ^ Moustafa et al. 2016, p. 728.
- ^ Palakurthi & Burugupally 2019, pp. 1–2.
- ^ Palakurthi & Burugupally 2019, pp. 1, 4.
- ^ Moustafa et al. 2016, pp. 727–728.
- ^ Moustafa et al. 2016, p. 731.
- ^ Mirelman et al. 2019, p. 1.
- ^ Moustafa et al. 2016, p. 734.
- ^ Moustafa et al. 2016, p. 732.
- ^ Moustafa et al. 2016, p. 733.
- ^ Aarslanda & Krambergera 2015, pp. 660, 662.
- ^ Aarslanda & Krambergera 2015, pp. 659–660.
- ^ Weintraub & Mamikonyan 2019, p. 661.
- ^ Aarslanda & Krambergera 2015, p. 660.
- ^ a b Biundo, Weis & Antonini 2016, p. 1.
- ^ a b Gonzalez-Latapi et al. 2021, p. 74.
- ^ Palma & Kaufmann 2020, pp. 372–373.
- ^ Pfeiffer 2020, p. 1464.
- ^ a b c Palma & Kaufmann 2020, p. 373.
- ^ Palma & Kaufmann 2020, pp. 1465–1466.
- ^ Pfeiffer 2020, p. 1467.
- ^ Han et al. 2022, p. 2.
- ^ Pfeiffer 2020, p. 1468.
- ^ Pfeiffer 2020, pp. 1471–1473.
- ^ a b Zhu et al. 2016, p. 685.
- ^ Corrà et al. 2023, pp. 225–226.
- ^ Zhu et al. 2016, p. 688.
- ^ Zhu et al. 2016, p. 687.
- ^ Weil et al. 2016, pp. 2831–2832.
- ^ Weil et al. 2016, p. 2828.
- ^ Stefani & Högl 2020, p. 121.
- ^ Dodet et al. 2024, p. 1.
- ^ Bollu & Sahota 2017, pp. 381–382.
- ^ Niemann, Billnitzer & Jankovic 2021, p. 61.
- ^ Almikhlafi 2024, p. 7.
- ^ a b c d e f g h i j k Morris et al. 2024.
- ^ a b c Toffoli, Vieira & Schapira 2020, p. 1.
- ^ a b Brundin & Melki 2017, p. 9808.
- ^ Ho & Wing 2024, pp. 1–2.
- ^ Toffoli, Vieira & Schapira 2020, p. 2.
- ^ a b Salles, Tirapegui & Chaná-Cuevas 2024, p. 2.
- ^ Farrow et al. 2024, p. 1.
- ^ Bandres-Cigaa et al. 2020, p. 2.
- ^ a b c d Tanner & Ostrem 2024.
- ^ Toffoli, Vieira & Schapira 2020, pp. 1–2.
- ^ Smith & Schapira 2022, pp. 1–15.
- ^ a b De Mirandaa et al. 2024, p. 46.
- ^ Dorsey & Bloem 2024, pp. 453–454.
- ^ a b c d Dorsey & Bloem 2024, p. 454.
- ^ Bloem & Boonstra 2023, p. e948–e949.
- ^ a b Rietdijk et al. 2017, p. 1.
- ^ Dorsey & Bloem 2024, pp. 453–455.
- ^ Langston 2017, p. S14.
- ^ Santos-Lobato, p. 1.
- ^ Wu & Schekman 2024, p. 1.
- ^ Brundin & Melki 2017, p. 9809.
- ^ Vázquez-Vélez & Zoghbi 2021, p. 96.
- ^ Dickson, 2018 & S31.
- ^ Wu & Schekman 2024, pp. 1–2.
- ^ Brundin & Melki 2017, p. 9812.
- ^ a b Dorsey et al., p. 363.
- ^ a b Rietdijk et al. 2017, p. 2.
- ^ Rietdijk et al. 2017, p. 3.
- ^ Dorsey et al., pp. 363–364, 371–372.
- ^ Goldstein 2020, p. 169.
- ^ Goldstein 2021, pp. 1–3.
- ^ Chen, Turnbull & Reeve 2019, pp. 1, 15.
- ^ Chen, Turnbull & Reeve 2019, pp. 1, 4–5, 15.
- ^ Chen, Turnbull & Reeve 2019, p. 2.
- ^ Borsche et al. 2021, p. 45.
- ^ Chen, Turnbull & Reeve 2019, p. 2, 13.
- ^ Chen, Turnbull & Reeve 2019, pp. 6–7, 8, 15.
- ^ Borsche et al. 2021, pp. 47–49.
- ^ Tan et al. 2020, p. 303.
- ^ Tan et al. 2020, p. 304.
- ^ Pardo-Moreno et al. 2023, p. 3.
- ^ a b c Vázquez-Vélez & Zoghbi 2021, p. 88.
- ^ a b Dickson 2018, p. S32.
- ^ a b Ye et al. 2023, p. 98.
- ^ Vázquez-Vélez & Zoghbi 2021, p. 93.
- ^ a b Henderson, Trojanowski & Lee 2019, p. 2.
- ^ Ye et al. 2023, p. 96.
- ^ a b Chen, Gu & Wang 2022.
- ^ Menšíková et al. 2022, p. 8.
- ^ a b Borghammer 2018, p. 5.
- ^ Vázquez-Vélez & Zoghbi 2021, p. 95.
- ^ Vázquez-Vélez & Zoghbi 2021, p. 89.
- ^ Menšíková et al. 2022, p. 6.
- ^ a b Vázquez-Vélez & Zoghbi 2021, pp. 96–99.
- ^ Vázquez-Vélez & Zoghbi 2021, pp. 96–97.
- ^ Vázquez-Vélez & Zoghbi 2021, pp. 98–99.
- ^ Vázquez-Vélez & Zoghbi 2021, p. 99.
- ^ Vázquez-Vélez & Zoghbi 2021, p. 100.
- ^ Ye et al. 2023, p. 112.
- ^ Ascherio & Schwarzschild 2016, p. 1257.
- ^ Coleman & Martin 2022, pp. 2321–2322.
- ^ Ascherio & Schwarzschild 2016, p. 1260.
- ^ Delic et al. 2020, pp. 1–2.
- ^ a b c d Ascherio & Schwarzschild 2016, p. 1259.
- ^ a b c d Crotty & Schwarzschild 2020, p. 1.
- ^ a b c Fabbri et al. 2024, p. 2.
- ^ Ascherio & Schwarzschild 2016, p. 1262.
- ^ Grotewolda & Albina 2024, pp. 1–2.
- ^ a b Grotewolda & Albina 2024, p. 2.
- ^ Rose, Schwarzschild & Gomperts 2024, pp. 268–269.
- ^ a b Grotewolda & Albina 2024, p. 3.
- ^ Ren & Chen 2020, p. 1.
- ^ Singh, Tripathi & Singh 2021, p. 10.
- ^ Ascherio & Schwarzschild 2016, pp. 1265–1266.
- ^ Lin et al. 2024, p. 1.
- ^ Ascherio & Schwarzschild 2016, p. 1263.
- ^ Ascherio & Schwarzschild 2016, p. 1261.
- ^ Kamal et al. 2020, p. 8.
- ^ a b c Jankovic J (April 2008). "Parkinson's disease: clinical features and diagnosis". Journal of Neurology, Neurosurgery, and Psychiatry. 79 (4): 368–376. doi:10.1136/jnnp.2007.131045. PMID 18344392. Archived from the original on 19 August 2015.
- ^ a b The National Collaborating Centre for Chronic Conditions, ed. (2006). "Diagnosing Parkinson's Disease". Parkinson's Disease. London: Royal College of Physicians. pp. 29–47. ISBN 978-1-8601-6283-1. Archived from the original on 24 September 2010.
- ^ a b Armstrong & Okun 2020. sfn error: multiple targets (2×): CITEREFArmstrongOkun2020 (help)
- ^ Poewe W, Wenning G (November 2002). "The differential diagnosis of Parkinson's disease". European Journal of Neurology. 9 (Suppl 3): 23–30. doi:10.1046/j.1468-1331.9.s3.3.x. PMID 12464118.
- ^ Gibb WR, Lees AJ (June 1988). "The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease". Journal of Neurology, Neurosurgery, and Psychiatry. 51 (6): 745–752. doi:10.1136/jnnp.51.6.745. PMC 1033142. PMID 2841426.
- ^ Tanner CM, Ostrem JL (August 2024). "Parkinson's Disease". New England Journal of Medicine. 391 (5): 442–452. doi:10.1056/NEJMra2401857. PMID 39083773.
- ^ Mustafa HI, Fessel JP, Barwise J, Shannon JR, Raj SR, Diedrich A, et al. (January 2012). "Dysautonomia: perioperative implications". Anesthesiology. 116 (1): 205–215. doi:10.1097/ALN.0b013e31823db712. PMC 3296831. PMID 22143168.
- ^ Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G (February 2016). "Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis". Neurology. 86 (6): 566–576. doi:10.1212/WNL.0000000000002350. PMID 26764028. S2CID 207110404.
- ^ a b c d Brooks DJ (April 2010). "Imaging approaches to Parkinson disease". Journal of Nuclear Medicine. 51 (4): 596–609. doi:10.2967/jnumed.108.059998. PMID 20351351.
- ^ Schwarz ST, Afzal M, Morgan PS, Bajaj N, Gowland PA, Auer DP (2014). "The 'swallow tail' appearance of the healthy nigrosome - a new accurate test of Parkinson's disease: a case-control and retrospective cross-sectional MRI study at 3T". PLOS ONE. 9 (4): e93814. Bibcode:2014PLoSO...993814S. doi:10.1371/journal.pone.0093814. PMC 3977922. PMID 24710392.
- ^ Mahlknecht P, Krismer F, Poewe W, Seppi K (April 2017). "Meta-analysis of dorsolateral nigral hyperintensity on magnetic resonance imaging as a marker for Parkinson's disease". Movement Disorders. 32 (4): 619–623. doi:10.1002/mds.26932. PMID 28151553. S2CID 7730034.
- ^ Cho SJ, Bae YJ, Kim JM, Kim D, Baik SH, Sunwoo L, et al. (March 2021). "Diagnostic performance of neuromelanin-sensitive magnetic resonance imaging for patients with Parkinson's disease and factor analysis for its heterogeneity: a systematic review and meta-analysis". European Radiology. 31 (3): 1268–1280. doi:10.1007/s00330-020-07240-7. PMID 32886201. S2CID 221478854.
- ^ Boonstra JT, Michielse S, Temel Y, Hoogland G, Jahanshahi A (February 2021). "Neuroimaging Detectable Differences between Parkinson's Disease Motor Subtypes: A Systematic Review". Movement Disorders Clinical Practice. 8 (2): 175–192. doi:10.1002/mdc3.13107. PMC 7853198. PMID 33553487.
- ^ Suwijn SR, van Boheemen CJ, de Haan RJ, Tissingh G, Booij J, de Bie RM (2015). "The diagnostic accuracy of dopamine transporter SPECT imaging to detect nigrostriatal cell loss in patients with Parkinson's disease or clinically uncertain parkinsonism: a systematic review". EJNMMI Research. 5: 12. doi:10.1186/s13550-015-0087-1. PMC 4385258. PMID 25853018.
- ^ "DaTSCAN Approval Letter" (PDF). FDA.gov. Food and Drug Administration. Retrieved 22 March 2019.
- ^ a b Armstrong MJ, Okun MS (February 2020). "Diagnosis and Treatment of Parkinson Disease: A Review". JAMA. 323 (6): 548–560. doi:10.1001/jama.2019.22360. PMID 32044947. S2CID 211079287.
- ^ a b c d Greenland & Barker 2018.
- ^ McCann H, Stevens CH, Cartwright H, Halliday GM (January 2014). "α-Synucleinopathy phenotypes". Parkinsonism & Related Disorders. 20 (Suppl 1): S62–S67. doi:10.1016/S1353-8020(13)70017-8. hdl:1959.4/53593. PMID 24262191.
- ^ Ganguly J, Jog M (5 November 2020). "Tauopathy and Movement Disorders-Unveiling the Chameleons and Mimics". Frontiers in Neurology. 11: 599384. doi:10.3389/fneur.2020.599384. PMC 7674803. PMID 33250855.
- ^ Gupta D, Kuruvilla A (December 2011). "Vascular parkinsonism: what makes it different?". Postgraduate Medical Journal. 87 (1034): 829–836. doi:10.1136/postgradmedj-2011-130051. PMID 22121251. S2CID 29227069.
- ^ Miguel-Puga A, Villafuerte G, Salas-Pacheco J, Arias-Carrión O (22 September 2017). "Therapeutic Interventions for Vascular Parkinsonism: A Systematic Review and Meta-analysis". Frontiers in Neurology. 8: 481. doi:10.3389/fneur.2017.00481. PMC 5614922. PMID 29018399.
- ^ Levin J, Kurz A, Arzberger T, Giese A, Höglinger GU (February 2016). "The Differential Diagnosis and Treatment of Atypical Parkinsonism". Deutsches Ärzteblatt International. 113 (5): 61–69. doi:10.3238/arztebl.2016.0061. PMC 4782269. PMID 26900156.
- ^ Simon, Greenberg & Aminoff 2017, p. page number needed.
- ^ a b c Connolly & Lang 2014.
- ^ de Bie et al. 2020, p. 3.
- ^ a b de Bie et al. 2020, pp. 1, 3.
- ^ a b c Kobylecki 2020, p. 395.
- ^ de Bie et al. 2020, p. 4.
- ^ a b c Limousin & Foltynie 2019, p. 234.
- ^ a b Bronstein et al. 2011, p. 169.
- ^ Tambasco, Romoli & Calabresi 2018, p. 1239.
- ^ LeWitt & Fahn 2016, p. S5-S6.
- ^ Tambasco, Romoli & Calabresi 2018, pp. 1239–1240.
- ^ Tambasco, Romoli & Calabresi 2018, p. 1240.
- ^ Leta et al. 2023, p. 1466.
- ^ Leta et al. 2022, pp. 1466–1468.
- ^ Tambasco, Romoli & Calabresi 2018, p. 1241.
- ^ Leta et al. 2022, p. 1468.
- ^ Jing et al. 2023, p. 1224.
- ^ de Bie et al. 2020, pp. 1, 3–4.
- ^ a b Jing et al. 2023, p. 1225.
- ^ a b Jing et al. 2023, p. 1226.
- ^ Kobylecki 2020, p. 396.
- ^ a b de Bie et al. 2020, p. 1.
- ^ Robakis & Fahn 2015, pp. 433–434.
- ^ Robakis & Fahn 2015, p. 433.
- ^ Binde et al. 2018, p. 1924.
- ^ Tan, Jenner & Chen 2022, p. 477.
- ^ a b c Alborghetti & Nicoletti 2019.
- ^ Robakis & Fahn 2015, p. 435.
- ^ The National Collaborating Centre for Chronic Conditions.
- ^ Seppi et al. 2019, pp. 183, 185, 188.
- ^ Elbers et al. 2015.
- ^ Rissardo et al. 2022, p. 1.
- ^ Lozano et al., pp. 1–2.
- ^ Lozano et al., p. 2.
- ^ a b Bronstein et al. 2011, p. 165.
- ^ Lozano et al., p. 6.
- ^ Moosa et al. 2019, pp. 1244–1249.
- ^ Bronstein et al. 2011, p. 168.
- ^ Bronstein et al. 2011, p. 166.
- ^ Tofani et al. 2020, p. 891.
- ^ a b Ernst et al. 2023.
- ^ Crotty & Schwarzschild 2020, pp. 1–2.
- ^ a b Ahlskog 2011, p. 292.
- ^ Costa et al. 2024.
- ^ Ramazzina, Bernazzoli & Costantino 2017, pp. 620–623.
- ^ O'Sullivan & Schmitz 2007, pp. 873, 876.
- ^ O'Sullivan & Schmitz 2007, p. 880.
- ^ O'Sullivan & Schmitz 2007, p. 879.
- ^ McDonnell et al. 2018, pp. 607–609.
- ^ Pu et al. 2021, pp. 1–2.
- ^ Tofani et al. 2020, pp. 891, 900.
- ^ Lister 2020, pp. 99–100.
- ^ a b Barichella, Cereda & Pezzoli 2009, pp. 1888.
- ^ Barichella, Cereda & Pezzoli 2009, pp. 1887.
- ^ Pasricha, Guerrero-Lopez & Kuo 2024, p. 212.
- ^ Pasricha, Guerrero-Lopez & Kuo 2024, p. 216.
- ^ a b Ghoche 2012, pp. S2–S3.
- ^ Wilcox 2010, p. 26.
- ^ Ferrell et al. 2007, p. 741.
- ^ Ghoche 2012, p. S3.
- ^ Casey 2013, pp. 20–22.
- ^ Bernat & Beresford 2013, pp. 135, 137, 138.
- ^ a b c d Corcoran & Kluger 2021, p. 956.
- ^ Fereshtehnejad et al. 2017, p. 1967.
- ^ Tolosa et al. 2021, p. 385.
- ^ Dommershuijsen et al. 2023, pp. 2–3.
- ^ Murueta-Goyena, Muiño & Gómez-Esteban 2017, p. 26.
- ^ Murueta-Goyena, Muiño & Gómez-Esteban 2017, p. 27.
- ^ Caballol, Martí & Tolosa 2007, p. S358.
- ^ Murueta-Goyena, Muiño & Gómez-Esteban 2024, p. 395.
- ^ Atalar, Oguz & Genc 2023, p. 163.
- ^ Chua et al. 2024, p. 1.
- ^ Corcoran, Muiño & Kluger 2021, p. 1.
- ^ Ben-Shlomo et al. 2024, p. 283.
- ^ Varden, Walker & O'Callaghan 2024, p. 1.
- ^ Zhu et al. 2024, p. e464.
- ^ a b Ben-Shlomo et al. 2024, p. 286.
- ^ Deliz, Tanner & Gonzalez-Latapi 2024, p. 166.
- ^ a b c Ben-Shlomo et al. 2024, p. 284.
- ^ Dorsey et al. 2018, p. S4.
- ^ a b Deliz, Tanner & Gonzalez-Latapi 2024, p. 165.
- ^ a b c d Ben-Shlomo et al. 2024, p. 285.
- ^ Li et al. 2019, p. 1.
- ^ a b Deliz, Tanner & Gonzalez-Latapi 2024, pp. 164–165.
- ^ Huang et al. 2024, pp. 1–2.
- ^ Lewis et al. 2020, p. 389.
- ^ Goetz 2011, pp. 1–2.
- ^ Lees 2007, p. S327.
- ^ a b c Goetz 2011, p. 2.
- ^ a b Louis 1997, p. 1069.
- ^ a b c d e Lees 2007, p. S328.
- ^ Lees 2007, p. S329.
- ^ Bereczki 2010, p. 290.
- ^ a b Blonder 2018, pp. 3–4.
- ^ Bereczki 2010, pp. 290–293.
- ^ Blonder 2018, p. 3.
- ^ Sousa-Santos, Pozzobon & Teixeira 2024, pp. 1–2.
- ^ Lees 2007, p. S331.
- ^ Fahn 2008, p. S500—S501, S504–S505.
- ^ Polymeropoulos et al. 1997.
- ^ Schulz-Schaeffer 2010, p. 131.
- ^ Lanska 2010, p. 507.
- ^ Guridi & Lozano 1997, pp. 1180–1183.
- ^ Fahn 2008, p. S497.
- ^ Fahn 2008, p. S501.
- ^ Coffey 2009, pp. 209–210.
- ^ Prenger et al. 2020, p. 2.
- ^ Crooks et al. 2023, p. 2,7.
- ^ Crooks et al. 2023, p. 2.
- ^ a b Schiess et al. 2022, p. 931.
- ^ a b c Yang et al. 2020, p. 1.
- ^ a b Schiess et al. 2022, p. 933.
- ^ Schiess et al. 2022, p. 929.
- ^ Schiess et al. 2022, p. 930.
- ^ Lees 2007, pp. S327–S334
- ^ GlaxoSmithKline.
- ^ National Parkinson Foundation.
- ^ Time 1960.
- ^ Parkinson's Disease Foundation.
- ^ American Parkinson Disease Association.
- ^ European Parkinson's Disease Association.
- ^ Parkinson's Foundation.
- ^ The Michael J. Fox Foundation for Parkinson's Research.
- ^ Davis 2007.
- ^ Brockes 2010.
- ^ Burleson 2023.
- ^ Brey 2006.
- ^ Matthews 2006, p. 10–23.
- ^ Macur 2008.
- ^ Davis Phinney Foundation.
- ^ Luca et al. 2018, pp. 1–2.
- ^ Gerstenbrand 2007, p. 121.
- ^ McCrum 2017.
- ^ Kinsley 2014.
- ^ Raudino 2011, pp. 945–949.
- ^ Gupta et al. 2015, pp. 1447–1452.
- ^ Boettcher et al. 2015, p. E8.
- ^ Gerstenbrand 2007, pp. 121–125.
- ^ Glass 2016.
- ^ Poewe et al. 2017.
- ^ Pardo-Moreno et al. 2023, p. 1.
- ^ Li & Le 2020, p. 183.
- ^ Heinzel et al. 2019.
- ^ a b Pardo-Moreno et al. 2023, pp. 12–13.
- ^ a b Alfaidi, Barker & Kuan 2024, p. 1.
- ^ Jasutkar, Oh & Mouradian 2022, p. 208.
- ^ Pardo-Moreno et al. 2023, pp. 10–11.
- ^ Pardo-Moreno et al. 2023, p. 13.
- ^ a b Pardo-Moreno et al. 2023, p. 10.
- ^ Parmar, Grealish & Henchcliffe 2020, pp. 103.
- ^ Parmar, Grealish & Henchcliffe 2020, pp. 103–104.
- ^ Parmar, Grealish & Henchcliffe 2020, pp. 106.
- ^ Henchcliffe & Parmar 2018, pp. 134.
- ^ Parmar, Grealish & Henchcliffe 2020, pp. 106, 108.
- ^ Schweitzer et al. 2020, p. 1926.
- ^ Parmar, Grealish & Henchcliffe 2020, pp. 105, 109.
- ^ Henchcliffe & Parmar 2018, pp. 132.
- ^ Van Laar et al. 2021, p. S174.
- ^ Hitti et al. 2019, p. 16.
- ^ Hitti et al. 2019, pp. 16–17.
- ^ Van Laar et al. 2021, p. S174, S176.
- ^ Hitti et al. 2019, p. 21.
- ^ Shaheen et al. 2024, pp. 5–6.
Works cited
[edit]Books
[edit]- Bhattacharyya KB (2017). "Chapter One - Hallmarks of Clinical Aspects of Parkinson's Disease Through Centuries". In Bhatia KP, Chaudhuri KR, Stamelou M (eds.). Parkinson's Disease. International Review of Neurobiology. pp. 1–23.
- Cooper G, Eichhorn G, Rodnitzky RL (2008). "Parkinson's disease". In Conn PM (ed.). Neuroscience in medicine. Humana Press. ISBN 978-1-6032-7454-8.
- Dissanayaka NN (8 March 2021). "Chapter 9: Anxiety in Parkinson's Disease". In Byrne GJ, Panchana NA (eds.). Anxiety in Older People: Clinical and Research Perspectives. Cambridge University Press. pp. 139–156. doi:10.1017/9781139087469.009. ISBN 978-1-1088-2636-5. S2CID 87250745.
- Ferri FF (2010). "Chapter P". Ferri's differential diagnosis: a practical guide to the differential diagnosis of symptoms, signs, and clinical disorders (2nd ed.). Elsevier/Mosby. ISBN 978-0-3230-7699-9.
- Lanska DJ (2010). "Chapter 33: The history of movement disorders". Handbook of Clinical Neurology. 3. Vol. 95. History of Neurology. pp. 501–546. doi:10.1016/S0072-9752(08)02133-7. ISBN 978-0-444-52009-8. PMID 19892136.
- O'Sullivan SB, Schmitz TJ (2007). "Parkinson's Disease". Physical Rehabilitation (5th ed.). F.A. Davis. ISBN 978-0-8036-1247-1.
- Simon RP, Greenberg D, Aminoff MJ (2017). Lange Clinical Neurology (10th ed.). McGraw-Hill. ISBN 978-1-2598-6172-7.
- Stoker TB, Greenland JC, eds. (December 2018). Parkinson's Disease: Pathogenesis and Clinical Aspects. Codon Publications. ISBN 978-0-9944-3816-4.
- Dallapiazza RF, De Vloo PD, Fomenko A, Lee DJ, Hamani C, Munhoz RP, et al. (2018). "Chapter 8: Considerations for Patient and Target Selection in Deep Brain Stimulation surgery for Parkinson's disease". In Stoker TB, Greenland JC (eds.). Parkinson's disease: Pathogenesis and Clinical Aspects. Codon Publications. doi:10.15586/codonpublications.parkinsonsdisease.2018.ch8. ISBN 978-0-9944-3816-4. PMID 30702838. S2CID 81155324.
- Greenland JC, Barker RA (2018). "Chapter 6: The Differential Diagnosis of Parkinson's Disease". In Stoker TB, Greenland JC (eds.). Parkinson's disease: Pathogenesis and Clinical Aspects. Codon Publications. pp. 109–128. doi:10.15586/codonpublications.parkinsonsdisease.2018.ch6. ISBN 978-0-9944-3816-4. PMID 30702839. S2CID 80908095.
- Stoker TB, Torsney KM, Barker RA (2018). "Chapter 3: Pathological mechanisms and clinical aspects of GBA1 mutation-associated Parkinson's disease". In Stoker TB, Greenland JC (eds.). Parkinson's Disease: Pathogenesis and clinical aspects. pp. 45–64. doi:10.15586/codonpublications.parkinsonsdisease.2018.ch3. ISBN 978-0-9944-3816-4. PMID 30702840. S2CID 92170834.
- Tolosa E, Jankovic E, eds. (2007). Parkinson's disease and movement disorders. Lippincott Williams & Wilkins. ISBN 978-0-7817-7881-7.
- Dickson DV (2007). "Neuropathology of movement disorders". In Tolosa E, Jankovic JJ (eds.). Parkinson's disease and movement disorders. Lippincott Williams & Wilkins. ISBN 978-0-7817-7881-7.
- Fung VS, Thompson PD (2007). "Rigidity and spasticity". In Tolosa E, Jankovic E (eds.). Parkinson's disease and movement disorders. Lippincott Williams & Wilkins. ISBN 978-0-7817-7881-7.
- Tolosa E, Katzenschlager R (2007). "Pharmacological management of Parkinson's disease". In Tolosa E, Jankovic JJ (eds.). Parkinson's disease and movement disorders. Lippincott Williams & Wilkins. ISBN 978-0-7817-7881-7.
- Truong DD, Bhidayasiri R (2016). "50: Parkinson's disease". In Lisak RP, Truong DD, Carroll WM, Bhidayasiri R (eds.). International Neurology. John Wiley & Sons. ISBN 978-1-1187-7736-7.
- Vertes AC, Beato MR, Sonne J, Khan Suheb MZ (June 2023). "Parkinson-Plus Syndrome". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 36256760. Retrieved 2 May 2024.
- Lorenzl S, Nubling G, Perrar KM, Voltz (August 2013). "Palliative treatment of chronic neurologic disorders". In Bernat JL, Beresford R (eds.). Handbook of Clinical Neurology. Vol. 118. pp. 133–139. PMID 24182372.
- The National Collaborating Centre for Chronic Conditions, ed. (2006). "Non-motor features of Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 113–133. ISBN 978-1-8601-6283-1. Archived from the original on 24 September 2010.
Journal articles
[edit]- Binde CD, Tvete IF, Gåsemyr J, Natvig B, Klemp M (September 2018). "A multiple treatment comparison meta-analysis of monoamine oxidase type B inhibitors for Parkinson's disease". British Journal of Clinical Pharmacology. 84 (9): 1917–1927. doi:10.1111/bcp.13651. PMC 6089809. PMID 29847694.
- Caballol N, Martí MJ, Tolosa E (September 2007). "Cognitive dysfunction and dementia in Parkinson disease". Mov. Disord. 22 (Suppl 17): S358–66. doi:10.1002/mds.21677. PMID 18175397. S2CID 3229727.
- Henchcliffe C, Parmar M (2018). "Repairing the Brain: Cell Replacement Using Stem Cell-Based Technologies". Journal of Parkinson's Disease. 8 (s1): S131–S137. doi:10.3233/JPD-181488. PMC 6311366. PMID 30584166.
- Panicker N, Ge P, Dawson VL, Dawson TM (April 2021). "The cell biology of Parkinson's disease". The Journal of Cell Biology. 220 (4). doi:10.1083/jcb.202012095. PMC 8103423. PMID 33749710.
- Parmar M, Grealish S, Henchcliffe C (February 2020). "The future of stem cell therapies for Parkinson disease". Nature Reviews. Neuroscience. 21 (2): 103–115. doi:10.1038/s41583-019-0257-7. PMID 31907406.
- Tolosa E, Garrido A, Scholz SW, Poewe W (May 2021). "Challenges in the diagnosis of Parkinson's disease". The Lancet. Neurology. 20 (5): 385–397. doi:10.1016/S1474-4422(21)00030-2. PMC 8185633. PMID 33894193.
- Blauwendraat C, Nalls MA, Singleton AB (February 2020). "The genetic architecture of Parkinson's disease". The Lancet. Neurology. 19 (2): 170–178. doi:10.1016/S1474-4422(19)30287-X. PMC 8972299. PMID 31521533.
- Winiker K, Kertscher B (2023). "Behavioural interventions for swallowing in subjects with Parkinson's disease: A mixed methods systematic review". International Journal of Language & Communication Disorders. 58 (4): 1375–1404. doi:10.1111/1460-6984.12865. PMID 36951546.
- Islam MS, Azim F, Saju H, Zargaran A, Shirzad M, Kamal M, et al. (September 2021). "Pesticides and Parkinson's disease: Current and future perspective". Journal of Chemical Neuroanatomy. 115: 101966. doi:10.1016/j.jchemneu.2021.101966. PMC 8842749. PMID 33991619.
- Hansen D, Ling H, Lashley T, Holton JL, Warner TT (April 2019). "Review: Clinical, neuropathological and genetic features of Lewy body dementias". Neuropathology and Applied Neurobiology. 45 (7): 635–654. doi:10.1111/nan.12554. PMID 30977926.
- Wallace ER, Segerstrom SC, van Horne CG, Schmitt FA, Koehl LM (2022). "Meta-Analysis of Cognition in Parkinson's Disease Mild Cognitive Impairment and Dementia Progression". Neuropsychology Review. 32 (1): 149–160. doi:10.1007/s11065-021-09502-7. PMID 33860906.
- Dolgacheva LP, Zinchenko VP, Goncharov NV (2022). "Molecular and Cellular Interactions in Pathogenesis of Sporadic Parkinson Disease". International Journal of Molecular Sciences. 23 (21): 13043. doi:10.3390/ijms232113043. PMC 9657547. PMID 36361826.
- Leta V, Urso D, Batzu L, Lau YH, Mathew D, Boura I, et al. (2022). "Viruses, parkinsonism and Parkinson's disease: the past, present and future". Journal of Neural Transmission. 129 (9): 1119–1132. doi:10.1007/s00702-022-02536-y. PMC 9422946. PMID 36036863.
- Limphaibool N, Iwanowski P, Holstad MJ, Kobylarek D, Kozubski W (2019). "Infectious Etiologies of Parkinsonism: Pathomechanisms and Clinical Implications". Frontiers in Neurology. 10: 652. doi:10.3389/fneur.2019.00652. PMC 6593078. PMID 31275235.
- Bologna M, Truong D, Jankovic J (2022). "The etiopathogenetic and pathophysiological spectrum of parkinsonism". Journal of the Neurological Sciences. 433: 1–8. doi:10.1016/j.jns.2021.120012. PMID 34642022.
- Prajjwal P, Kolanu ND, Reddy YB, Ahmed A, Marsool MD, Santoshi K, et al. (2024). "Association of Parkinson's disease to Parkinson's plus syndromes, Lewy body dementia, and Alzheimer's dementia". Health Science Reports. 7 (4): e2019. doi:10.1002/hsr2.2019. PMC 10982460. PMID 38562616.
- Olfatia N, Shoeibia A, Litvanb I (2019). "Progress in the treatment of Parkinson-Plus syndromes". Parkinsonism & Related Disorders. 59: 101–110. doi:10.1016/j.parkreldis.2018.10.006. PMID 30314846.
- Calabresi P, Mechelli A, Natale G, Volpicelli-Daley L, Di Lazzaro G, Ghiglieri V (2023). "Alpha-synuclein in Parkinson's disease and other synucleinopathies: from overt neurodegeneration back to early synaptic dysfunction". Cell Death & Disease. 14 (3): 176. doi:10.1038/s41419-023-05672-9. PMC 9977911. PMID 36859484.
- Ramesh SD, Arachchige AS (2023). "Depletion of dopamine in Parkinson's disease and relevant therapeutic options: A review of the literature". AIMS Neuroscience. 10 (3): 200–231. doi:10.3934/Neuroscience.2023017. PMC 10567584. PMID 37841347.
- Ascherio A, Schwarzschild MA (2016). "The epidemiology of Parkinson's disease: risk factors and prevention". Lancet Neurology. 15 (12): 1257–1272. doi:10.1016/S1474-4422(16)30230-7. PMID 27751556.
- Crotty GF, Schwarzschild MA (2020). "Chasing Protection in Parkinson's Disease: Does Exercise Reduce Risk and Progression?". Frontiers in Aging Neuroscience. 12: 186. doi:10.3389/fnagi.2020.00186. PMC 7318912. PMID 32636740.
- Singh A, Tripathi P, Singh S (2021). "Neuroinflammatory responses in Parkinson's disease: relevance of Ibuprofen in therapeutics". Inflammopharmacology. 29 (1): 5–14. doi:10.1007/s10787-020-00764-w. PMID 33052479.
- Fabbri M, Rascol O, Foltynie T, Carroll C, Postuma RB, Porcher R, et al. (2024). "Advantages and Challenges of Platform Trials for Disease Modifying Therapies in Parkinson's Disease". Movement Disorders. 39 (9): 1468–1477. doi:10.1002/mds.29899. PMID 38925541.
- Kamal H, Tan GC, Ibrahim SF, Shaikh MF, Mohamed IN, Mohamed RM, et al. (2020). "Alcohol Use Disorder, Neurodegeneration, Alzheimer's and Parkinson's Disease: Interplay Between Oxidative Stress, Neuroimmune Response and Excitotoxicity". Frontiers in Cellular Neuroscience. 14: 282. doi:10.3389/fncel.2020.00282. PMC 7488355. PMID 33061892.
- Lin J, Pang D, Li C, Ou R, Yu Y, Cui Y, et al. (2024). "Calcium channel blockers and Parkinson's disease: a systematic review and meta-analysis". Therapeutic Advances in Neurological Disorders. 17: 1–8. doi:10.1177/17562864241252713. PMC 11104025. PMID 38770432.
- Grotewolda N, Albina RL (2024). "Update: Protective and risk factors for Parkinson disease". Parkinsonism and Related Disorders. 125: 1–12. doi:10.1016/j.parkreldis.2024.107026. PMID 38879999.
- Rose KN, Schwarzschild MS, Gomperts SN (2024). "Clearing the Smoke: What Protects Smokers from Parkinson's Disease?". Movement Disorders. 39 (2): 267–272. doi:10.1002/mds.29707. PMC 10923097. PMID 38226487.
- Ren X, Chen J (2020). "Caffeine and Parkinson's Disease: Multiple Benefits and Emerging Mechanisms". Frontiers in Neuroscience. 14: 1–12. doi:10.3389/fnins.2020.602697. PMC 7773776. PMID 33390888.
- Ben-Shlomo Y, Darweesh S, Llibre-Guerra J, Marras C, Luciano MS, Tanner C (2024). "The epidemiology of Parkinson's disease". The Lancet. 403 (10423): 283–292. doi:10.1016/S0140-6736(23)01419-8. PMC 11123577. PMID 38245248.
- Deliz JR, Tanner CM, Gonzalez-Latapi P (2024). "Epidemiology of Parkinson's Disease: An Update". Current Neurology and Neuroscience Reports. 24 (6): 163–179. doi:10.1007/s11910-024-01339-w. PMID 38642225.
- Dorsey ER, Sherer T, Okun MS, Bloem BR (2018). "The Emerging Evidence of the Parkinson Pandemic". Journal of Parkinson's Disease. 8 (s1): S3–S8. doi:10.3233/JPD-181474. PMC 6311367. PMID 30584159.
- Li G, Ma J, Cui S, He Y, Xiao Q, Liu J, et al. (2019). "Parkinson's disease in China: a forty-year growing track of bedside work". Translational Neurodegeneration. 8 (1): 22. doi:10.1186/s40035-019-0162-z. PMC 6668186. PMID 31384434.
- Varden R, Walker R, O'Callaghan A (2024). "No trend to rising rates: A review of Parkinson's prevalence studies in the United Kingdom". Parkinsonism & Related Disorders. 128: 1–6. doi:10.1016/j.parkreldis.2024.107015. PMID 38876845.
- Zhu J, Cui Y, Zhang J, Yan R, Su D, Zhao D, et al. (2024). "Temporal trends in the prevalence of Parkinson's disease from 1980 to 2023: a systematic review and meta-analysis". The Lancet: Healthy Longetivity. 5: e464–e479. doi:10.1016/j.parkreldis.2024.107015. PMID 38876845.
- Goetz CG (2011). "The history of Parkinson's disease: early clinical descriptions and neurological therapies". Cold Spring Harbor Perspectives in Medicine. 1 (1): a008862. doi:10.1101/cshperspect.a008862. PMC 3234454. PMID 22229124.
- Lees AJ (2007). "Unresolved issues relating to the shaking palsy on the celebration of James Parkinson's 250th birthday". Movement Disorders. 22 (S17): S327–S334. doi:10.1002/mds.21684. PMID 18175393.
- Louis ED (1997). "The shaking palsy, the first forty-five years: a journey through the British literature". Movement Disorders. 12 (6): 1068–1072. doi:10.1002/mds.870120638. PMID 9399240.
- Lewis PA, Plun-Favreau H, Rowley M, Spillane J (2020). "Pierre D. and the first photographs of Parkinson's disease". Movement Disorders. 35 (3): 389–391. doi:10.1002/mds.27965. PMC 7155099. PMID 31975439.
- Bereczki D (2010). "The description of all four cardinal signs of Parkinson's disease in a Hungarian medical text published in 1690". Parkinsonism & Related Disorders. 16 (4): 290–293. doi:10.1016/j.parkreldis.2009.11.006. PMID 19948422.
- Blonder LX (2018). "Historical and cross-cultural perspectives on Parkinson's disease". Journal of Complementary and Integrative Medicine. 15 (3): 1–15. doi:10.1515/jcim-2016-0065. PMID 29738310.
- Coffey RJ (2009). "Deep brain stimulation devices: a brief technical history and review". Artificial Organs. 33 (3): 208–220. doi:10.1111/j.1525-1594.2008.00620.x. PMID 18684199.
- Sousa-Santos PE, Pozzobon PM, Teixeira IL (2024). "Frederic Lewy: how the two World Wars changed his life, work, and name". Arquivos de Neuro-Psiquiatri. 82 (3): 001–002. doi:10.1055/s-0044-1779692. PMC 10927365. PMID 38467394.
- Fahn S (2008). "The history of dopamine and levodopa in the treatment of Parkinson's disease". Movement Disorders. 23 (S3): S497–S508. doi:10.1002/mds.22028. PMID 18781671.
- Schulz-Schaeffer WJ (2010). "The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia". Acta Neuropathologica. 120 (2): 131–143. doi:10.1007/s00401-010-0711-0. PMC 2892607. PMID 20563819.
- Guridi J, Lozano AM (1997). "A brief history of pallidotomy". Neurosurgery. 41 (5): 1169–1180. doi:10.1097/00006123-199711000-00029. PMID 9361073.
- Tolosa E, Garrido A, Scholz SW, Poewe W (May 2021). "Challenges in the diagnosis of Parkinson's disease". Lancet Neurology. 20 (5): 385–397. doi:10.1016/S1474-4422(21)00030-2. PMC 8185633. PMID 33894193.
- Corcoran J, Kluger BM (September 2023). "Prognosis in chronic progressive neurologic disease: a narrative review". Annals of Palliative Medicine. 12 (5): 952–962. doi:10.21037/apm-22-1338. PMID 37691335.
- Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB (July 2017). "Clinical criteria for subtyping Parkinson's disease: biomarkers and longitudinal progression". Brain. 140 (7): 1959–1976. doi:10.1093/brain/awx118. PMID 28549077.
- Dommershuijsen LJ, Darweesh SK, Ben-Shlomo Y, Kluger BM, Bloem BR (October 2023). "The elephant in the room: critical reflections on mortality rates among individuals with Parkinson's disease". npj Parkinson's Disease. 9 (1): 145. doi:10.1038/s41531-023-00588-9. PMC 10587193. PMID 37857675.
- Murueta-Goyena A, Muiño O, Gómez-Esteban JC (April 2024). "Prognostic factors for falls in Parkinson's disease: a systematic review". Acta Neurologica Belgica. 124 (2): 395–406. doi:10.1007/s13760-023-02428-2. PMC 10965733. PMID 38015306.
- Murueta-Goyena A, Muiño O, Gómez-Esteban JC (March 2017). "Dementia in Parkinson's disease". Journal of the Neurological Sciences. 374: 26–31. doi:10.1016/j.jns.2017.01.012. PMID 28088312.
- Chua WY, Wang JD, Chan CK, Chan L, Tan E (September 2024). "Risk of aspiration pneumonia and hospital mortality in Parkinson disease: A systematic review and meta-analysis". European Journal of Neurology. 31 (12): e16449. doi:10.1111/ene.16449. PMC 11555015. PMID 39236309.
- Won JH, Byun SJ, Oh B, Park SJ, Seo HG (September 2007). "Cognitive dysfunction and dementia in Parkinson disease". Scientific Reports. 22 (S17): S358–S366. doi:10.1002/mds.21677. PMID 18175397.
- Corcoran J, Kluger BM (2021). "Risk and mortality of aspiration pneumonia in Parkinson's disease: a nationwide database study". Scientific Reports. 11 (1): 6597. Bibcode:2021NatSR..11.6597W. doi:10.1038/s41598-021-86011-w. PMC 7988066. PMID 33758213.
- Atalar MS, Oguz O, Genc G (2023). "Hypokinetic Dysarthria in Parkinson's Disease: A Narrative Review". The Medical Bulletin of Sisle Etfal Hospital. 57 (2): 163–170. doi:10.14744/SEMB.2023.29560. PMC 10600629. PMID 37899809.
- Huang M, Bargues-Carot A, Riaz Z, Wickham H, Zenitsky G, Jin H, et al. (September 2022). "Impact of Environmental Risk Factors on Mitochondrial Dysfunction, Neuroinflammation, Protein Misfolding, and Oxidative Stress in the Etiopathogenesis of Parkinson's Disease". International Journal of Molecular Sciences. 23 (10808): 10808. doi:10.3390/ijms231810808. PMC 9505762. PMID 36142718.
- Gerstenbrand F, Karamat E (2007). "Adolf Hitler's Parkinson's disease and an attempt to analyse his personality structure". European Journal of Neurology. 6 (2): 121–127. doi:10.1111/j.1468-1331.1999.tb00003.x. PMID 10053222.
- Luca A, Nicoletti A, Mostile G, Zappia M (2018). "The Parkinsonian Personality: More Than Just a "Trait"". Frontiers in Neurology. 9 (1191): 1191. doi:10.3389/fneur.2018.01191. PMC 6340987. PMID 30697187.
- Rana AQ, Ahmed US, Chaudry ZM, Vasan S (May 2015). "Parkinson's disease: a review of non-motor symptoms". Expert Reviews Neurotherapeutics. 15 (5): 549–462. doi:10.1586/14737175.2015.1038244. PMID 25936847.
- Biundo R, Weis L, Antonini A (September 2016). "Cognitive decline in Parkinson's disease: the complex picture". npj Parkinson's Disease. 2 (16018): 16018. doi:10.1038/npjparkd.2016.18. PMC 5516581. PMID 28725699.
- Gonzalez-Latapi P, Bayram E, Litvan I, Marras C (May 2021). "Cognitive Impairment in Parkinson's Disease: Epidemiology, Clinical Profile, Protective and Risk Factors". Behavioral Sciences. 11 (5): 74. doi:10.3390/bs11050074. PMC 8152515. PMID 34068064.
- Zhu M, Li M, Ye D, Jiang W, Lei T, Shu K (March 2016). "Sensory symptoms in Parkinson's disease: Clinical features, pathophysiology, and treatment". Journal of Neuroscience Research. 94 (8): 685–692. doi:10.1002/jnr.23729. PMID 26948282.
- Corrà MF, Vila-Chã N, Sardoeira A, Hansen C, Sousa AP, Reis I, et al. (January 2023). "Peripheral neuropathy in Parkinson's disease: prevalence and functional impact on gait and balance". Brain. 146 (1): 225–236. doi:10.1093/brain/awac026. PMC 9825570. PMID 35088837.
- Weil RS, Schrag AE, Warren JD, Crutch SJ, Lees AJ, Morris HR (July 2016). "Visual dysfunction in Parkinson's disease". Brain. 146 (139): 2827–2843. doi:10.1093/brain/aww175. PMC 5091042. PMID 27412389.
- Pfeiffer RF (October 2020). "Autonomic Dysfunction in Parkinson's Disease". Neurotherapeutics. 17 (4): 1464–1479. doi:10.1007/s13311-020-00897-4. PMC 7851208. PMID 32789741.
- Palma JA, Kaufmann H (March 2018). "Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies". Movement Disorders. 33 (3): 372–390. doi:10.1002/mds.27344. PMC 5844369. PMID 29508455.
- Han MN, Finkelstein DI, McQuade RM, Diwakarla S (January 2022). "Gastrointestinal Dysfunction in Parkinson's Disease: Current and Potential Therapeutics". Journal of Personalized Medicine. 12 (2): 144. doi:10.3390/jpm12020144. PMC 8875119. PMID 35207632.
- Aarslanda D, Krambergera MG (2015). "Neuropsychiatric Symptoms in Parkinson's Disease". Journal of Personalized Medicine. 5 (3): 659–667. doi:10.3233/JPD-150604. PMID 26406147.
- Niemann N, Billnitzer A, Jankovic J (January 2021). "Parkinson's disease and skin". Parkinsonism & Related Disorders. 82: 61–76. doi:10.1016/j.parkreldis.2020.11.017. PMID 33248395.
- Almikhlafi MA (January 2024). "A review of the gastrointestinal, olfactory, and skin abnormalities in patients with Parkinson's disease". Neurosciences. 29 (1): 4–9. doi:10.17712/nsj.2024.1.20230062 (inactive 1 November 2024). PMC 10827020. PMID 38195133.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - Stefani A, Högl B (January 2020). "Sleep in Parkinson's disease". Neuropsychopharmacology. 45 (1): 121–128. doi:10.1038/s41386-019-0448-y. PMC 6879568. PMID 31234200.
- Bollu PC, Sahota P (2017). "Sleep and Parkinson Disease". Missouri Medicine. 114 (5): 381–386. PMC 6140184. PMID 30228640.
- Dodet P, Houot M, Leu-Semenescu S, Corvol JC, Lehéricy S, Mangone G, et al. (February 2024). "Sleep disorders in Parkinson's disease, an early and multiple problem". npj Parkinson's Disease. 10 (1): 46. doi:10.1038/s41531-024-00642-0. PMC 10904863. PMID 38424131.
- Moustafa AA, Chakravarthy S, Phillips JR, Gupta A, Keri S, Polner B, et al. (September 2016). "Motor symptoms in Parkinson's disease: A unified framework". Neuroscience & Biobehavioral Reviews. 68: 727–740. doi:10.1016/j.neubiorev.2016.07.010. PMID 27422450.
- Mirelman A, Bonato P, Camicioli R, Ellis TD, Giladi N, Hamilton JL, et al. (April 2019). "Gait impairments in Parkinson's disease". Lancet Neurology. 17 (7): 697–708. doi:10.1016/S1474-4422(19)30044-4. PMID 30975519.
- Sveinbjornsdottir S (October 2016). "The clinical symptoms of Parkinson's disease". Journal of Neurochemistry. 139 (Suppl 1): 318–324. doi:10.1111/jnc.13691. PMID 27401947.
- Abusrair AH, Elsekaily W, Bohlega S (13 September 2022). "Tremor in Parkinson's Disease: From Pathophysiology to Advanced Therapies". Tremor and Other Hyperkinetic Movements. 12 (1): 29. doi:10.5334/tohm.712. PMC 9504742. PMID 36211804.
- Bologna M, Paparella G, Fasano A, Hallett M, Berardelli A (December 2019). "Evolving concepts on bradykinesia". Brain. 143 (3): 727–750. doi:10.1093/brain/awz344. PMC 8205506. PMID 31834375.
- Ferreira-Sánchez MD, Moreno-Verdú M, Cano-de-la-Cuerda R (February 2020). "Quantitative Measurement of Rigidity in Parkinson's Disease: A Systematic Review". Sensors. 20 (3): 880. Bibcode:2020Senso..20..880F. doi:10.3390/s20030880. PMC 7038663. PMID 32041374.
- Palakurthi B, Burugupally SP (September 2019). "Postural Instability in Parkinson's Disease: A Review". Brain Sciences. 9 (239): 239. doi:10.3390/brainsci9090239. PMC 6770017. PMID 31540441.
- Yang W, Hamilton JL, Kopil C, Beck JC, Tanner CM, Albin RL, et al. (July 2020). "Current and projected future economic burden of Parkinson's disease in the U.S." npj Parkinson's Disease. 6: 15. doi:10.1038/s41531-020-0117-1. PMC 7347582. PMID 32665974.
- Cunha M, Almeida H, Guimarães I, Ferreira LN (July 2020). "Current and projected future economic burden of Parkinson's disease in the U.S.". Journal of Public Health.
- Schiess N, Cataldi R, Okun MS, Fothergill-Misbah N, Dorsey ER, Bloem BR, et al. (September 2022). "Six Action Steps to Address Global Disparities in Parkinson Disease: A World Health Organization Priority" (PDF). JAMA. 79 (9): 929–936. doi:10.1001/jamaneurol.2022.1783. PMID 35816299.
- Prenger MT, Madray R, Van Hedger K, Anello M, MacDonald PA (2020). "Social Symptoms of Parkinson's Disease". Parkinson's Disease. 2020: 8846544. doi:10.1155/2020/8846544. PMC 7790585. PMID 33489081.
- Crooks S, Carter G, Wilson CB, Wynne L, Stark P, Doumas M, et al. (2023). "Exploring public perceptions and awareness of Parkinson's disease: A scoping review". PLOS ONE. 18 (9): e0291357. Bibcode:2023PLoSO..1891357C. doi:10.1371/journal.pone.0291357. PMC 10503766. PMID 37713383.
- Luca A, Nicoletti A, Mostile G, Zappia M (2018). "The Parkinsonian Personality: More Than Just a "Trait"". Frontiers in Neurology. 9 (1191): 1191. doi:10.3389/fneur.2018.01191. PMC 6340987. PMID 30697187.
- Raudino F (2011). "The Parkinson disease before James Parkinson". History of Neurology. 33: 945–949.
- Gupta R, Kim C, Agarwal N, Lieber B, Monaco EA (2015). "Understanding the Influence of Parkinson Disease on Adolf Hitler's Decision-Making during World War II". World Neurosurgery. 84 (5): 1447–1452. doi:10.1016/j.wneu.2015.06.014. PMID 26093359.
- Boettcher L, Bonney P, Smitherman A, Sughrue M (2015). "Hitler's parkinsonism". Neurosurgical Focus. 39 (1): E8. doi:10.3171/2015.4.FOCUS1563. PMID 26126407.
- Brey RL (April 2006). "Muhammad Ali's Message: Keep Moving Forward". Neurology Now. 2 (2): 8. doi:10.1097/01222928-200602020-00003. Archived from the original on 27 September 2011. Retrieved 22 August 2020.
- Matthews W (April 2006). "Ali's Fighting Spirit". Neurology Now. 2 (2): 10–23. doi:10.1097/01222928-200602020-00004. S2CID 181104230.
- Dorsey ER, Bloem BR (January 2024). "Parkinson's Disease Is Predominantly an Environmental Disease". Journal of Parkinson's Disease. 14 (3): 103–115. doi:10.3233/JPD-230357. PMC 11091623. PMID 38217613.
- Bandres-Ciga S, Diez-Fairen M, Kim JJ, Singleton AB (April 2020). "Genetics of Parkinson's disease: An introspection of its journey towards precision medicine". Neurobiology of Disease. 137: 1–9. doi:10.1016/j.nbd.2020.104782. PMC 7064061. PMID 31991247.
- Toffoli M, Vieira SR, Schapira AH (June 2020). "Genetic causes of PD: A pathway to disease modification". Neuropharmacology. 170: 1–13. doi:10.1016/j.neuropharm.2020.108022. PMID 32119885.
- Dorsey ER, Zafar M, Lettenberger SE, Pawlik ME, Kinel D, Frissen M, et al. (2023). "Trichloroethylene: An Invisible Cause of Parkinson's Disease?". Journal of Parkinson's Disease. 13 (2): 203–218. doi:10.3233/JPD-225047. PMC 10041423. PMID 36938742.
- Chen C, Turnbull DM, Reeve AK (May 2019). "Mitochondrial Dysfunction in Parkinson's Disease—Cause or Consequence?". Biology. 8 (2): 38. doi:10.3390/biology8020038. PMC 6627981. PMID 31083583.
- Morris HR, Spillantini MG, Sue CM, Williams-Gray CH (January 2024). "The pathogenesis of Parkinson's disease". Lancet. 403 (10423): 293–304. doi:10.1016/s0140-6736(23)01478-2. PMID 38245249.
- Gogna T, Housden BE, Houldsworth A (September 2024). "Exploring the Role of Reactive Oxygen Species in the Pathogenesis and Pathophysiology of Alzheimer's and Parkinson's Disease and the Efficacy of Antioxidant Treatment". Antioxidants. 13 (1138): 1138. doi:10.3390/antiox13091138. PMC 11429442. PMID 39334797.
- Brundin P, Melki R (October 2017). "Prying into the Prion Hypothesis for Parkinson's Disease". Journal of Neuroscience. 37 (41): 9808–9818. doi:10.1523/JNEUROSCI.1788-16.2017. PMC 5637113. PMID 29021298.
- Salles PA, Tirapegui JM, Chaná-Cuevas P (22 March 2024). "Genetics of Parkinson's disease: Dominant forms and GBA". Neurology Perspectives. 4 (3): 100153. doi:10.1016/j.neurop.2024.100153.
- Farrow SL, Gokuladhas S, Schierding W, Pudjihartono M, Perry JK, Cooper AA, et al. (October 2024). "Identification of 27 allele-specific regulatory variants in Parkinson's disease using a massively parallel reporter assay". npj Parkinson's Disease. 10 (1): 44. doi:10.1038/s41531-024-00659-5. PMC 10899198. PMID 38413607.
- Smith L, Schapira AH (April 2022). "GBA Variants and Parkinson Disease: Mechanisms and Treatments". Cells. 11 (8): 1261. doi:10.3390/cells11081261. PMC 9029385. PMID 35455941.
- Goldstein DS (February 2020). "The catecholaldehyde hypothesis: where MAO fits in". Journal of Neural Transmission. 127 (2): 169–177. doi:10.1007/s00702-019-02106-9. PMC 10680281. PMID 31807952.
- Goldstein DS (June 2021). "The Catecholaldehyde Hypothesis for the Pathogenesis of Catecholaminergic Neurodegeneration: What We Know and What We Do Not Know". International Journal of Molecular Sciences. 22 (11): 5999. doi:10.3390/ijms22115999. PMC 8199574. PMID 34206133.
- Santos-Lobato BL (April 2024). "Towards a methodological uniformization of environmental risk studies in Parkinson's disease". npj Parkinson's Disease. 10 (1): 86. doi:10.1038/s41531-024-00709-y. PMC 11024193. PMID 38632283.
- De Mirandaa BR, Goldmanb SM, Millerc GW, Greenamyred JT, Dorseye ER (April 2024). "Preventing Parkinson's Disease: An Environmental Agenda". Journal of Parkinson's Disease. 12 (1): 45–68. doi:10.3233/JPD-212922. PMC 8842749. PMID 34719434.
- Langston JW (March 2017). "The MPTP Story". Journal of Parkinson's Disease. 7 (1): S11–S19. doi:10.3233/JPD-179006. PMC 5345642. PMID 28282815.
- Dorsey ER, De Mirandab BR, Horsager J, Borghammer P (April 2024). "The Body, the Brain, the Environment, and Parkinson's Disease". Journal of Parkinson's Disease. 14 (3): 363–381. doi:10.3233/JPD-240019. PMC 11091648. PMID 38607765.
- Bloem BR, Boonstra TA (December 2023). "The inadequacy of current pesticide regulations for protecting brain health: the case of glyphosate and Parkinson's disease". The Lancet. Planetary Health. 7 (12): e948–e949. doi:10.1016/s2542-5196(23)00255-3. PMID 37949088.
- Delic V, Beck KD, Pang KC, Citron BA (April 2020). "Biological links between traumatic brain injury and Parkinson's disease". Acta Neuropathologica Communications. 8 (1): 45. doi:10.1186/s40478-020-00924-7. PMC 7137235. PMID 32264976.
- Coleman C, Martin I (16 December 2022). "Unraveling Parkinson's Disease Neurodegeneration: Does Aging Hold the Clues?". Journal of Parkinson's Disease. 12 (8): 2321–2338. doi:10.3233/JPD-223363. PMC 9837701. PMID 36278358.
- Wu S, Schekman RW (September 2024). "Intercellular transmission of alpha-synuclein". Frontiers in Molecular Neuroscience. 17: 1–12. doi:10.3389/fnmol.2024.1470171. PMC 11422390. PMID 39324117.
- Ho H, Wing SS (November 2024). "α-Synuclein ubiquitination – functions in proteostasis and development of Lewy bodies". Frontiers in Molecular Neuroscience. 17: 1–19. doi:10.3389/fnmol.2024.1498459. PMC 11588729. PMID 39600913.
- Rietdijk CD, Perez-Pardo P, Garssen J, van Wezel RJ, Kraneveld AD (February 2017). "Exploring Braak's Hypothesis of Parkinson's Disease". Frontiers in Neurology. 8: 37. doi:10.3389/fneur.2017.00037. PMC 5304413. PMID 28243222.
- Borsche M, Pereira SL, Klein C, Grünewald A (February 2021). "Mitochondria and Parkinson's Disease: Clinical, Molecular, and Translational Aspects". Journal of Parkinson's Disease. 11 (1): 45–60. doi:10.3233/JPD-201981. PMC 7990451. PMID 33074190.
- Tan E, Chao Y, West A, Chan L, Poewe W, Jankovic J (April 2020). "Parkinson disease and the immune system - associations, mechanisms and therapeutics". Nature Reviews Neurology. 16 (6): 303–318. doi:10.1038/s41582-020-0344-4. PMID 32332985.
- Kobylecki C (July 2020). "Update on the diagnosis and management of Parkinson's disease". Clinical Medicine. 20 (4): 393–398. PMID 32675145.
- de Bie RM, Clarke CE, Espay AJ, Fox SH, Lang AE (March 2020). "Initiation of pharmacological therapy in Parkinson's disease: when, why, and how". Lancet Neurology. 19 (5): 452–461. PMID 32171387.
- Ferrell B, Connor SR, Cordes A, Dahlin CM, Fine PG, Hutton N, et al. (June 2007). "The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum". Journal of Pain and Symptom Management. 33 (6): 737–744. doi:10.1016/j.jpainsymman.2007.02.024. PMID 17531914.
- Lorenzl S, Nübling G, Perrar KM, Voltz R (2013). "Palliative treatment of chronic neurologic disorders". Ethical and Legal Issues in Neurology. Handbook of Clinical Neurology. Vol. 118. Elsevier. pp. 133–139. doi:10.1016/B978-0-444-53501-6.00010-X. ISBN 978-0-4445-3501-6. PMID 24182372.
- Ghoche R (December 2012). "The conceptual framework of palliative care applied to advanced Parkinson's disease". Parkinsonism & Related Disorders. 18 (Suppl 3): S2–S5. doi:10.1016/j.parkreldis.2012.06.012. PMID 22771241.
- Wilcox SK (January 2010). "Extending palliative care to patients with Parkinson's disease". British Journal of Hospital Medicine. 71 (1): 26–30. doi:10.12968/hmed.2010.71.1.45969. PMID 20081638.
- Moens K, Higginson IJ, Harding R (October 2014). "Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review". Journal of Pain and Symptom Management. 48 (4): 660–677. doi:10.1016/j.jpainsymman.2013.11.009. PMID 24801658.
- Casey G (August 2013). "Parkinson's disease: a long and difficult journey". Nursing New Zealand. 19 (7): 20–24. PMID 24195263.
- Lister T (May 2020). "Nutrition and Lifestyle Interventions for Managing Parkinson's Disease: A Narrative Review". Journal of Movement Disorders. 13 (2): 97–104. PMID 32498495.
- Barichella M, Cereda E, Pezzoli G (October 2009). "Major nutritional issues in the management of Parkinson's disease". Movement Disorders. 24 (13): 1881–1892. PMID 19691125.
- Pasricha TS, Guerrero-Lopez IL, Kuo B (March 2024). "Management of Gastrointestinal Symptoms in Parkinson's Disease: A Comprehensive Review of Clinical Presentation, Workup, and Treatment". Movement Disorders. 58 (3): 211–220. PMID 38260966.
- McDonnell MN, Rischbieth B, Schammer TT, Seaforth C, Shaw AJ, Phillips AC (May 2018). "Lee Silverman Voice Treatment (LSVT)-BIG to improve motor function in people with Parkinson's disease: a systematic review and meta-analysis". Clinical Rehabilitation. 32 (5): 607–618. PMID 28980476.
- Pu T, Huang M, Kong X, Wang M, Chen X, Feng X, et al. (December 2021). "Lee Silverman Voice Treatment to Improve Speech in Parkinson's Disease: A Systemic Review and Meta-Analysis". Parkinson's Disease. 2021: 1–10. PMID 35070257.
- Tofani M, Ranieri A, Fabbrini G, Berardi A, Pelosin E, Valente D, et al. (October 2020). "Efficacy of Occupational Therapy Interventions on Quality of Life in Patients with Parkinson's Disease: A Systematic Review and Meta-Analysis". Movement Disorders. 7 (8): 891–901. PMID 33163559.
- Ernst M, Folkerts AK, Gollan R, Lieker E, Caro-Valenzuela J, Adams A, et al. (1 January 2023). "Physical exercise for people with Parkinson's disease: a systematic review and network meta-analysis". The Cochrane Database of Systematic Reviews. 2024 (4): CD013856. doi:10.1002/14651858.CD013856.pub3. PMC 9815433. PMID 38588457.
- Ahlskog JE (July 2011). "Does vigorous exercise have a neuroprotective effect in Parkinson disease?". Neurology. 77 (3): 288–294. doi:10.1212/wnl.0b013e318225ab66. PMC 3136051. PMID 21768599.
- Costa V, Prati JM, de Oliveira BS, Brito TS, da Rocha F, Gianlorenço T, et al. (November 2024). "Physical Exercise for Treating the Anxiety and Depression Symptoms of Parkinson's Disease: Systematic Review and Meta-Analysis". Journal of Geriatric Psychiatry and Neurology. 37 (6): 415–435. doi:10.1177/08919887241237223. ISSN 0891-9887. PMID 38445606.
- Ramazzina I, Bernazzoli B, Costantino C (March 2017). "Systematic review on strength training in Parkinson's disease: an unsolved question". Clinical Interventions in Aging. 12: 619–628. doi:10.2147/CIA.S131903. PMC 5384725. PMID 28408811.
- Limousin P, Foltynie T (April 2019). "Long-term outcomes of deep brain stimulation in Parkinson disease". Nature Reviews Neurology. 14 (4): 234–242. PMID 30778210.
- Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. (February 2011). "Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues". Archives of Neurology. 68 (2): 165. doi:10.1001/archneurol.2010.260. PMC 4523130. PMID 20937936.
- Lozano CS, Tam J, Lozano AM (January 2018). "The changing landscape of surgery for Parkinson's Disease". Movement Disorders. 33 (1): 36–47. PMID 29194808.
- Connolly BS, Lang AE (April 2014). "Pharmacological treatment of Parkinson disease: a review". JAMA. 311 (16): 1670–1683. doi:10.1001/jama.2014.3654. PMID 24756517. S2CID 205058847.
- Moosa S, Martínez-Fernández R, Elias WJ, Del Alamo M, Eisenberg HM, Fishman PS (September 2019). "The role of high-intensity focused ultrasound as a symptomatic treatment for Parkinson's disease". Movement Disorders. 34 (9): 1243–1251. PMID 31291491.
- Tambasco N, Romoli M, Calabresi P (October 2018). "Levodopa in Parkinson's Disease: Current Status and Future Developments". Current Neuropharmacology. 16 (8): 1239–1252. PMID 28494719.
- LeWitt PA, Fahn S (April 2016). "Levodopa therapy for Parkinson disease: A look backward and forward". Neurology. 86 (14): S3–S12. PMID 28494719.
- Leta V, Klingelhoefer L, Longardner K, Campagnolo M, Levent HÇ, Aureli F, et al. (May 2023). "Gastrointestinal barriers to levodopa transport and absorption in Parkinson's disease". European Journal of Neurology. 30 (5): 1465–1480. PMID 36757008.
- Oertel WH (13 March 2017). "Recent advances in treating Parkinson's disease". F1000Research. 6: 260. doi:10.12688/f1000research.10100.1. PMC 5357034. PMID 28357055.
- Horowski R, Löschmann PA (February 2019). "Classical dopamine agonists". Journal of Neural Transmission. 126 (4): 449–454. PMID 30805732.
- Jing X, Yang H, Taximaimaiti R, Wang X (2023). "Advances in the Therapeutic Use of Non-Ergot Dopamine Agonists in the Treatment of Motor and Non-Motor Symptoms of Parkinson's Disease". Current Neuropharmacology. 21 (5): 1224–1240. PMID 36111769.
- Tan Y, Jenner P, Chen S (2022). "Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson's Disease: Past, Present, and Future". Journal of Parkinson's Disease. 12 (2): 477–493. PMID 34957948.
- Robakis D, Fahn S (June 2015). "Defining the Role of the Monoamine Oxidase-B Inhibitors for Parkinson's Disease". CNS Drugs. 29 (6): 433–441. PMID 26164425.
- Alborghetti M, Nicoletti F (2019). "Different Generations of Type-B Monoamine Oxidase Inhibitors in Parkinson's Disease: From Bench to Bedside". Current Neuropharmacology. 17 (9): 861–873. doi:10.2174/1570159X16666180830100754. PMC 7052841. PMID 30160213.
- Armstrong MJ, Okun MS (February 2020). "Diagnosis and Treatment of Parkinson Disease: A Review". JAMA. 323 (6): 548–560. doi:10.1001/jama.2019.22360. PMID 32044947. S2CID 211079287.
- Rissardo JP, Durante I, Sharon I, Caprara AL (September 2022). "Pimavanserin and Parkinson's Disease Psychosis: A Narrative Review". Brain Sciences. 23 (12): 1–11. PMID 36291220.
- Elbers RG, Verhoef J, van Wegen EE, Berendse HW, Kwakkel G (October 2015). "Interventions for fatigue in Parkinson's disease". The Cochrane Database of Systematic Reviews (Review). 2015 (10): CD010925. doi:10.1002/14651858.CD010925.pub2. PMC 9240814. PMID 26447539.
- Seppi K, Ray Chaudhuri K, Coelho M, Fox SH, Katzenschlager R, Perez Lloret S, et al. (February 2019). "Update on treatments for nonmotor symptoms of Parkinson's disease—an evidence-based medicine review". Movement Disorders. 34 (2): 180–198. doi:10.1002/mds.27602. PMC 6916382. PMID 30653247.
- Gouda NA, Elkamhawy A, Cho J (February 2022). "Emerging Therapeutic Strategies for Parkinson's Disease and Future Prospects: A 2021 Update". Biomedicines. 10 (2): 1–40. PMID 35203580.
- Jasutkar HG, Oh SE, Mouradian MM (January 2022). "Therapeutics in the Pipeline Targeting α-Synuclein for Parkinson's Disease". Pharmacological Reviews. 74 (1): 207–237. PMID 35017177.
- Pardo-Moreno T, García-Morales V, Suleiman-Martos S, Rivas-Domínguez A, Mohamed-Mohamed H, Ramos-Rodríguez JJ, et al. (February 2023). "Current Treatments and New, Tentative Therapies for Parkinson's Disease". Pharmaceutics. 15 (3): 1–24. PMID 36986631.
- Shaheen N, Shaheen A, Osama M, Nashwan AJ, Bharmauria V, Flouty O (October 2024). "MicroRNAs regulation in Parkinson's disease, and their potential role as diagnostic and therapeutic targets". npj Parkinson's Disease volume. 10 (3): 1–11. PMID 39369002.
- Van Laar AD, Van Laar VS, San Sebastian W, Merola A, Elder JB, Lonser RR, et al. (2021). "An Update on Gene Therapy Approaches for Parkinson's Disease: Restoration of Dopaminergic Function". Journal of Parkinson's Disease. 11 (S2): S173–S182. PMID 34366374.
- Schweitzer JS, Song B, Herrington TM, Park TY, Lee N, Ko S, et al. (May 2020). "Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson's Disease". The New England Journal of Medicine. 382 (20): 1926–1932. doi:10.1056/NEJMoa1915872. PMC 7288982. PMID 32402162.
- Alfaidi M, Barker RA, Kuan W (December 2024). "An update on immune-based alpha-synuclein trials in Parkinson's disease". Journal of Neurology. 272 (1): 1–9. PMID 39666171.
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. (March 2017). "Parkinson disease". Nature Reviews. Disease Primers. 3 (1): 17013. doi:10.1038/nrdp.2017.13. PMID 28332488. S2CID 11605091.
- Li T, Le W (February 2020). "Biomarkers for Parkinson's Disease: How Good Are They?". Neuroscience Bulletin. 36 (2): 183–194. PMID 31646434.
- Heinzel S, Berg D, Gasser T, Chen H, Yao C, Postuma RB (October 2019). "Update of the MDS research criteria for prodromal Parkinson's disease". Movement Disorders. 34 (10): 1464–1470. doi:10.1002/mds.27802. PMID 31412427. S2CID 199663713.
- Hitti FL, Yang AI, Gonzalez-Alegre P, Baltuch GH (September 2019). "Human gene therapy approaches for the treatment of Parkinson's disease: An overview of current and completed clinical trials". Parkinsonism & Related Disorders. 66: 16–24. doi:10.1016/j.parkreldis.2019.07.018. PMID 31324556. S2CID 198132349.
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. (27 June 1997). "Mutation in the α-Synuclein Gene Identified in Families with Parkinson's Disease". Science. 276 (5321): 2045–2047. doi:10.1126/science.276.5321.2045. ISSN 0036-8075. PMID 9197268.
- Zaman V, Shields DC, Shams R, Drasites KP, Matzelle D, Haque A, et al. (June 2021). "Cellular and molecular pathophysiology in the progression of Parkinson's disease". Metabolic Brain Disease. 36 (5): 815–827. PMID 33599945.
- Vázquez-Vélez GE, Zoghbi HY (July 2021). "Parkinson's Disease Genetics and Pathophysiology". Annual Review of Neuroscience. 44: 87–108. PMID 34236893.
- Warnecke T, Schäfer KH, Claus I, Del Tredici K, Jost WH (March 2022). "Gastrointestinal involvement in Parkinson's disease: pathophysiology, diagnosis, and management". npj parkinson's disease. 8: 1–13. PMID 35332158.
- Miller KM, Mercado NM, Sortwell CE (April 2021). "Synucleinopathy-associated pathogenesis in Parkinson's disease and the potential for brain-derived neurotrophic factor". npj parkinson's disease. 7 (1): 1–9. PMID 33846345.
- Borghammer P (January 2018). "How does parkinson's disease begin? Perspectives on neuroanatomical pathways, prions, and histology". Movement Disorders. 33 (1): 48–57. PMID 28843014.
- Zhang X, Gao F, Wang D, Li C, Fu Y, He W, et al. (October 2018). "Tau Pathology in Parkinson's Disease". Frontiers in Neurology. 9: 1–7. PMID 30333786.
- Henderson MX, Trojanowski JQ, Lee VM (September 2019). "α-Synuclein pathology in Parkinson's disease and related α-synucleinopathies". Neuroscience Letters. 709: 1–10. PMID 31170426.
- Ye H, Robak LA, Yu M, Cykowski M, Shulman JM (January 2023). "Genetics and Pathogenesis of Parkinson's Syndrome". Annual Review of Pathology: Mechanisms of Disease. 18: 95–121. PMID 36100231.
- Dickson DW (January 2018). "Neuropathology of Parkinson disease". Parkinsonism and Related Disorders. 46 (S1): S30–S33. PMID 28780180.
- Chen R, Gu X, Wang X (April 2022). "α-Synuclein in Parkinson's disease and advances in detection". Clinica Chimica Acta; International Journal of Clinical Chemistry. 529: 76–86. doi:10.1016/j.cca.2022.02.006. PMID 35176268.
- Menšíková K, Matěj R, Colosimo C, Rosales R, Tučková L, Ehrmann J, et al. (January 2022). "Lewy body disease or diseases with Lewy bodies?". npj Parkinson's Disease. 8 (1): 1-11. PMID 35013341.
- Koh J, Ito H (January 2017). "Differential diagnosis of Parkinson's disease and other neurodegenerative disorders". Nihon Rinsho. Japanese Journal of Clinical Medicine. 75 (1): 56–62. PMID 30566295.
- Ou Z, Pan J, Tang S, Duan D, Yu D, Nong H, et al. (7 December 2021). "Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson's Disease in 204 Countries/Territories From 1990 to 2019". Frontiers in Public Health. 9: 776847. doi:10.3389/fpubh.2021.776847. PMC 8688697. PMID 34950630.
Web sources
[edit]- "Parkinson's Disease". National Institute of Neurological Disorders and Stroke. Retrieved 2 September 2024.
- "Symptoms of PD". Stanford Parkinson's Community Outreach. Stanford University School Medicine. Retrieved 2 September 2024.
- Macur J (26 March 2008). "For the Phinney Family, a Dream and a Challenge". The New York Times. Archived from the original on 6 November 2014. Retrieved 25 May 2013.
About 1.5 million Americans have received a diagnosis of Parkinson's disease, but only 5 to 10 percent learn of it before age 40, according to the National Parkinson Foundation. Davis Phinney was among the few.
- "Who We Are". Davis Phinney Foundation. Archived from the original on 11 January 2012. Retrieved 18 January 2012.
- "Parkinson's – 'the shaking palsy'". GlaxoSmithKline. 1 April 2009. Archived from the original on 14 May 2011.
- "National Parkinson Foundation – Mission". Archived from the original on 21 December 2010. Retrieved 28 March 2011.
- "About PDF". Parkinson's Disease Foundation. Archived from the original on 15 May 2011. Retrieved 24 July 2016.
- "American Parkinson Disease Association: Home". American Parkinson Disease Association. Archived from the original on 10 May 2012. Retrieved 9 August 2010.
- "About EPDA". European Parkinson's Disease Association. 2010. Archived from the original on 15 August 2010. Retrieved 9 August 2010.
- "Notable Figures with Parkinson's". Parkinson's Foundation. Retrieved 22 November 2023.
- "Michael's Story". The Michael J. Fox Foundation for Parkinson's Research. Retrieved 7 May 2023.
News publications
[edit]- Burleson N, Breen K (9 November 2023). "Michael J. Fox talks funding breakthrough research for Parkinson's disease". CBS News. Retrieved 23 November 2023.
- Glass A (9 September 2016). "Mao Zedong dies in Beijing at age 82, Sept. 9, 1976". Politico. Retrieved 30 October 2023.
- Tauber P (17 July 1988). "Ali: Still Magic". The New York Times. Archived from the original on 17 November 2016. Retrieved 2 April 2011.
- McCrum R (20 November 2017). "The 100 best nonfiction books: No 94 – Leviathan by Thomas Hobbes (1651)". The Guardian. Retrieved 23 November 2023.
- Kinsley M (21 April 2014). "Have You Lost Your Mind?". The New Yorker. Retrieved 23 November 2023.
- "Education: Joy in Giving". Time. 18 January 1960. Archived from the original on 20 February 2011. Retrieved 2 April 2011.
- Davis P (3 May 2007). "Michael J. Fox". The Time 100. New York: Time. Archived from the original on 25 April 2011. Retrieved 2 April 2011.
- Brockes E (11 April 2009). "'It's the gift that keeps on taking'". The Guardian. Archived from the original on 8 October 2013. Retrieved 25 October 2010.










